
The wood-pasture hypothesis (also known as the Vera hypothesis and the megaherbivore theory) is a scientific hypothesis positing that open and semi-open pastures and wood-pastures formed the predominant type of landscape in post-glacial temperate Europe, rather than the common belief of primeval forests. The hypothesis proposes that such a landscape would be formed and maintained by large wild herbivores. Although others, including landscape ecologist Oliver Rackham, had previously expressed similar ideas, it was the Dutch researcher Frans Vera, who, in his 2000 book Grazing Ecology and Forest History, first developed a comprehensive framework for such ideas and formulated them into a theorem. Vera's proposals, although highly controversial, came at a time when the role grazers played in woodlands was increasingly being reconsidered, and are credited for ushering in a period of increased reassessment and interdisciplinary research in European conservation theory and practice. Although Vera largely focused his research on the European situation, his findings could also be applied to other temperate ecological regions worldwide, especially the broadleaved ones.
Vera's ideas have met with both rejection and approval in the scientific community, and continue to lay an important foundation for the rewilding-movement. While his proposals for widespread semi-open savanna as the predominant landscape of temperate Europe in the early to mid-Holocene have at large been rejected, they do partially agree with the established wisdom about vegetation structure during previous interglacials. Moreover, modern research has shown that, under the current climate, free-roaming large grazers can indeed influence and even temporarily halt vegetation succession. Whether the Holocene prior to the rise of agriculture provides an adequate approximation to a state of "pristine nature" at all has also been questioned, since by that time anatomically modern humans had already been omnipresent in Europe for millennia, with in all likelihood profound effects on the environment.
The severe loss of megafauna at the end of the Pleistocene and beginning of the Holocene known as the Quaternary extinction event, which is frequently linked to human activities, did not leave Europe unscathed and brought about a profound change in the European large mammal assemblage and thus ecosystems as a whole, which probably also affected vegetation patterns. The assumption, however, that the pre-Neolithic represents pristine conditions is a prerequisite for both the "high-forest theory" and the Vera hypothesis in their respective original forms. Whether or not the hypothesis is supported may thus further depend on whether or not the pre-Neolithic Holocene is accepted as a baseline for pristine nature, and thus also on whether the Quaternary extinction of megafauna is considered (primarily) natural or man-made.
Vera's hypothesis has important repercussions for nature conservation especially, because it advocates for a reorientation of emphasis away from the protection of old-growth forest (as per the competing high forest theory) and towards the conservation of open and semi-open grasslands and wood pastures, through extensive grazing. This aspect in particular has attracted considerable attention, and has made Vera's hypothesis an important point of reference for conservation grazing and rewilding initiatives. The wood-pasture hypothesis also has points of contact with traditional agricultural practices in Europe, which may conserve biodiversity in a similar way to wild herbivore herds.
Names and definitions
Frans Vera's hypothesis has many names, since Vera himself did not provide a distinguished name for it. Instead, he simply referred to it as the alternative hypothesis, alternative to the high-forest theory, which he called the null hypothesis.[1] As a result, it has been called by many names over the years, including the wood-pasture hypothesis,[2][3] the wooded pasture hypothesis,[4] the Vera hypothesis,[5] the temperate savanna hypothesis[4] and the open woodland hypothesis.[6] Especially in Continental Europe, it is commonly known as the megaherbivore hypothesis and literal translations of it.
Vera limited the geographic area of his ideas to Western and Central Europe between 45°N and 58°N latitude and 5°W and 25°E longitude. This includes most of the British Isles and everything between France (except the Southern third) and Poland and Southern Scandinavia to the Alps. Furthermore, he confined it to altitudes below 700 m (2,300 ft).[7] By extension, the North American East Coast is also addressed as an analogy with a comparable climate.[8]
High-forest theory

Heinrich Cotta: high-forest theory
In his 1817 work Anweisungen zum Waldbau (Directions for Silviculture), Heinrich Cotta posited that if humans abandoned his native Germany, in the space of 100 years it would be "covered with wood".[9] This assumption laid the foundation for what is now called the high-forest theory, which assumes that deciduous forests are the naturally predominant ecosystem type in the temperate, broad-leaved regions.
Frederic Clements: linear succession
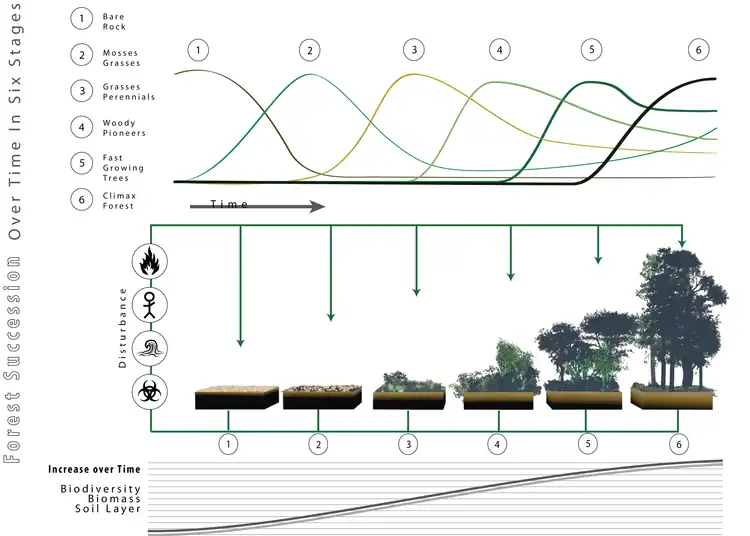
Later,[10] this position was accompanied by Clements' formulation of the theory of linear succession, meaning that under the right conditions bare ground would, over time, invariably become colonised by a succession of plant communities eventually leading to closed stands dominated by the tallest plant species. Because in most of the temperate hemisphere the potentially tallest plants are trees, the final product would therefore chiefly be forest. Albeit with changes in conceptualisation and some modifications, this concept remains the one favoured by most,[2] and provides the conceptual framework for many forest-related methods and customs in forestry and conservation. This includes the Prozessschutz doctrine advocated by German forest-ecologist Knut Sturm, which highlights the importance of non-intervention and space of time for forest protection, as it is implemented in forest reserves such as Białowieża.
Further refinements
Clements' notion of stable climax communities was later challenged and refined by authorities such as Arthur Tansley,[11] Alexander Watt[12] and Robert Whittaker,[13] who championed the inclusion of dynamic processes, like temporary collapse of canopy cover because of windthrow, fire or calamities, into Clements' framework. This, however, did not change anything about the status of the "high-forest theory" as the commonly accepted view; that without human intervention closed-canopy forest would dominate the global temperate regions as the potential natural vegetation. This is also the concept that was advocated by European plant experts like Heinz Ellenberg, Johannes Iversen and Franz Firbas.[14][15][16]
The reconstruction of vegetation history
Apart from theoretical considerations, this concept has relied and continues to rely heavily on both field observations and, more recently, on findings from pollen analysis, which allow inferences about the vegetation structure of past epochs. For example, vegetation trends can be reconstructed from the ratio of tree pollen to pollen associated with grassland. Pollen analysis is the most widely used means of generating historic vegetation data[17] an the analysis of pollen data has provided a solid database from which a predominance of forest throughout the early stages of the Holocene of temperate Europe, especially the Atlantic, is generally inferred,[18][19] although the possibility of regional differences remains open.[20][21] On that basis, the history of vegetation in Europe is generally reconstructed as a history of forest.[16][22][15]
Pollen analysis, however, has been criticized for its inherent bias towards wind-pollinated plant species and, importantly, wind-pollinated trees,[23] and has been shown to overestimate forest cover.[24][25] To account for this bias, a corrective model (REVEALS) is used,[6] whose application leads to results that differ substantially from those drawn from the traditional comparison of pollen percentages alone.[26] Alternatively to or in combination with pollen, fossil indicator organisms – such as beetles and molluscs – can be used to reconstruct vegetation structure.[20]
Large herbivores and high-forest theory

There is no general agreement on herbivores and their influence on succession in natural ecosystems in the temperate hemisphere. In the high-forest theory framework, wild herbivores are mostly considered as minor factors, derived from the assumption that the natural vegetation was forest. Therefore, wild herbivores were characterised by Tansley as followers of succession, not as actively influencing it, because otherwise Europe would not have been forested.[11] From this assumption the principle was developed that the natural abundance of herbivores does not hinder forest succession, which means that herbivore numbers are necessarily considered too high once as they impede natural forest regeneration. For example, WWF Russia considers five to seven animals the optimal density of bison per 1000 ha (10 km²), because if the population exceeds 13 animals per 1000 ha, first signs of vegetation suppression are observed.[27] Similarly, it is widely believed that two to seven deer per 1 square kilometre (1,000,000 m2) is a sustainable number[28] based on the assumption that if deer numbers exceed this bar, they start having a negative impact on woodland regeneration. Consequently, culling is commonly seen as necessary to reduce a perceived overabundance of deer to sustainable levels and mimic natural predation.[29][30][31]
Others, however, have criticised this view. In a 2023 publication, Brice B. Hanberry and Edward K. Faison argued that in the eastern United States, where white-tailed deer are commonly considered overabundant due to the extirpation of wolves and cougars, there are currently no more deer than there were historically when these predators were present. Furthermore, they found that even at densities that are perceived as too high, the influence of deer may be ecologically beneficial.[32] The assumption that population control through hunting is necessary in order to mimic the effect of natural predators is also not entirely supported by scientific analyses of natural predator-prey dynamics. Instead, the control of herbivore numbers in nature probably depends on other factors.[33][34][35] A perhaps more important influence predators may have on prey animals is the landscape of fear their presence can create, promoting landscape heterogeneity. However, in the presence of megafauna over 1,000 kilograms (0.98 long tons; 1.1 short tons), which are largely immune to predation, even this ability is limited.[36] Overall, how ungulate populations are controlled in nature is controversial, and food availability is an important constraint, even in the presence of apex predators.[35][37]
In regions with relatively intact large-mammal assemblages in Africa and Asia, as well as in European rewilding areas where "naturalistic grazing" is practised, herbivore biomass exceeds the values commonly deemed appropriate for temperate forests many times over. Here, herbivore biomass reaches a maximum of 16,000 kilograms (16 long tons; 18 short tons) per 1 square kilometre (0.39 sq mi), while the mammoth steppe with an estimated 10,500 kilograms (10.3 long tons; 11.6 short tons) per km2 falls within a similar range. The herbivore biomass of Britain during the Eemian interglacial has been estimated as more than 15,000 kilograms (15 long tons; 17 short tons) per km2, which is equivalent to more than 2.5 fallow deer per ha.[38] Hence, the ecologist Christopher Sandom and others have suggested that the comparatively high forest cover of the pre-Neolithic European Holocene may be a consequence of megaherbivore extinctions during the Quaternary extinction event, as compared to the last interglacial in Europe with a pristine megafauna, the Eemian, the early stages of the Holocene appear to have been much more forested. According to the authors, this is unlikely to be the result of the latter's only slightly cooler climate as compared to the Eemian.[3] However, this is also subject to debate.[39]
Background: grazers and browsers
The impact herbivores have on the landscape level depends on their way of feeding. Namely, browsers like roe deer, elk and the black rhino focus on woody vegetation, while the diet of grazers like horse, cattle and the white rhino is dominated by grasses and forbs. Intermediate feeders, like the wisent and the red deer, fall in between. Generally, grazers tend to be more social, less selective in their food choices and forage more intensively. Therefore, their impact on vegetation composition tends to be higher, as well as their ability to maintain open spaces.[40]
Since the extinction of the aurochs in 1627 and the wild horse around 1900, none of the remaining large wild herbivores in Europe is an obligate grazer. Similarly, domesticated descendants of aurochs and wild horse, cattle and horse, are now largely kept in stables, factory farms and close to settlements, making them effectively extinct in the landscape. What remains are browsers and mixed feeders[lower-alpha 1] – roe deer, red deer, elk, wild boar, wisent and beaver, often in low densities. Backbreeding-projects, such as the German Taurus project and the Dutch Tauros programme are addressing this issue by breeding domestic cattle that can be released into the landscape as hardy and sufficiently similar proxies to act as ecological replacements for the aurochs.[42] Similarly, primitive horse breeds such as the Konik, Exmoor pony and the Sorraia are being used as proxies for the tarpan.[43]
Frans Vera
_(28524158712).jpg.webp)
Vera argued that the dominating landscape-type of the early to mid-Holocene was not closed forest, but a semi-open, park-like one. This semi-open landscape, he proposed, was created and maintained by large herbivores. During the Holocene, these herbivores included aurochs, European bison, red deer and tarpan. Up to the Quaternary extinctions, many other megafaunal mammals like the straight-tusked elephant or Merck's rhinoceros existed in Europe as well, that probably kept the forests open during warm interglacial periods like the Eemian interglacial.[26] Vera also postulated that lowland forest did not emerge on a large scale before the onset of the Neolithic period and subsequent local extinctions of herbivores, which in turn allowed forests to thrive more unhindered. Indeed, investigations point to at least locally open circumstances, for example in floodplains, on infertile soils, chalklands and in submediterranean and continental areas, but maintain that forest largely dominated.[20]
In his book Vera also discussed the decline of ancient oak-hickory-forest communities in Eastern North America. Many forests that stem from Pre-Columbian times (old-growth forests) feature light-demanding oaks and hickories prominently. However, these do not readily regenerate in modern forests; a phenomenon commonly referred to as oak regeneration failure. Instead, shade-tolerant species such as red maple and American beech dominate increasingly. While the cause is still poorly understood, a lack of natural fire is commonly presumed to play a role.[44] Vera instead suggested that the grazing and browsing of wild herbivores, most importantly American bison, created the conditions oaks and hickories need for successful regeneration to happen, and explained the modern lack of regeneration of these species in forests with the mass-slaughter of bisons committed by European settlers.
Paleoecological evidence drawn from fossil Coleoptera deposits has also shown that, albeit rare, beetle species associated with grasslands and other open landscapes were present throughout the Holocene of Western Europe, which points to open habitats being present, but restricted.[45][46] However, paleoecological data from previous interglacials when the larger megafauna was still present indicate widespread warm temperate savannah. This could mean that elephants and rhinos were more effective creators of open landscapes than the herbivores left after the Quaternary extinction event.[3][26] On the other hand, traditional animal husbandry may have mitigated the effects of possibly human-induced megafaunal die-off, allowing the survival of species of the open landscape previously created and maintained by megafauna.[47]
Frans Vera was not the first to question the high-forest paradigm. Botanist Francis Rose had expressed doubts already in the 1960s, knowing about British plant and lichen species and their light requirements.[48] The relationship between large grazers and landscape openness, and the significance of the Quaternary extinctions of megafauna in this regard, had also been recognized prior to Vera. In 1992, for example, the archaeologist Wilhelm Schüle theorized that the genesis of closed forest in temperate Europe was the result of prehistoric man-made megafauna extinctions.[49] Landscape ecologist Oliver Rackham, in a 1998 article entitled "Savanna in Europe", envisaged a kind of savanna as the original predominant landscape type of northwestern Europe.[50] Vera, however, was the first to develop a comprehensive theorem to explain why forest did not dominate even in the Holocene, and to thus propose a real alternative to the high-forest theory.[48]
In some of its aspects, the wood-pasture hypothesis bears similarity to Gradmann's steppe theory[51] which was proposed by Robert Gradmann but challenged and refuted by scholars such as Reinhold Tüxen and Karl Bertsch.
Main arguments
Oak and hazel
Vera relies on several lines of argument based on experiments, ecology, evolutionary ecology, palynology, history and etymology. One of his main arguments is of an ecological nature; the widespread lack of successful regeneration of light-demanding tree species in modern forests. Especially the lack of regeneration of pedunculate oak, sessile oak (together hereafter addressed as "oak") and common hazel in Europe. He contrasts this reality with European pollen deposits from previous ages, where oak and hazel often form a dominant amount of pollen, making a dominance of these species in previous ages conceivable. Especially in regard to hazel, sufficient flowering is only achieved when enough sunlight is available, i.e. the plant grows outside of a closed canopy. He argues that the only explanation for the great abundance of oak and hazel pollen in previous ages is that the primeval landscape was open, and this contrast forms the principal theorem of his hypothesis. It has also been suggested that oak requires disturbances for successful establishment, disturbance large herbivores may provide.[52]
However, pollen records from islands that lacked many of the large grazers and browsers that, according to Vera, were essential for the maintenance of landscapes with an open character in temperate Europe show almost no differences in comparison to mainland Europe. More specifically, pollen records from Holocene Ireland, which during the early Holocene was apparently, owing to a lack of fossils, devoid of any big herbivores except for abundant wild boar and rare red deer, show almost equally high percentages of oak and hazel pollen. Thus it could be concluded that large herbivores were not a required factor for the degree of openness in a landscape, and that the abundance of pollen from species that are unable to reproduce and regenerate sufficiently under a closed canopy, such as hazel and oak, can only be explained by other factors like windthrow and natural fires.[53]
Vera's notion may be supported by observations over the course of 20 years forest regeneration in forest gaps created by windthrow, which showed that hornbeam and beech dominate the emerging stands and largely displace oaks on fertile, nutrient-rich soil.[54] However, after the last Ice Age oak returned earlier to Central and Western Europe than beech or hornbeam,[55] which may have contributed to its commonness, at least during the early Holocene. Still, other shade-tolerant tree species like lime and elm were equally fast returnees, and do not seem to have limited oak abundance.[55][56]
On the other hand, substantial natural oak-regeneration commonly takes place outside of forests in fringe and transitional habitats, suggesting that a focus on regeneration in forests in an attempt to explain oak regeneration failure may be insufficient in regard to the ecology of Central European oak species.[57] Rather, an underestimated reason for widespread failure of oak regeneration may be found in the direct effects of land-use changes since the early modern period, which has led to a more simplistic, homogeneous landscape,[57] as spontaneous regeneration of both oak and hazel does frequently occur in margins, thickets, and low-grazing-intensity or abandoned pasture/arable land.[58][59][60] Overall, oak is an adept coloniser of open areas and especially of transitional zones between vegetation zones such as forest and open grassland. Looking for regeneration within forests may therefore be futile from the outset. There is, therefore, no general "failure" in oak regeneration, but only a failure of oak regeneration within closed forests. This, however, may be expectable and natural given oak's colonising nature.[57]
Furthermore, new species of oak mildew (Erysiphe alphitoides) observed on European oaks for the first time at the beginning of the 20th century have been cited as a possible reason for the modern lack of oak regeneration in forests, since they affect the shade tolerance, particularly of young pedunculate and sessile oaks.[61] Although the origin of these new oak pathogens remains obscure, it seems to be an invasive species from the tropics, possibly conspecific with a pathogen found on mangos.[62]
 Oak sapling (left) growing in direct vicinity to a young blackthorn shrub.
Oak sapling (left) growing in direct vicinity to a young blackthorn shrub. Being relatively hardy to browsing, (e. g. by roe deer) oaks (shrub in the corner) are generally able to establish under moderate grazing pressure, depending on the conditions.
Being relatively hardy to browsing, (e. g. by roe deer) oaks (shrub in the corner) are generally able to establish under moderate grazing pressure, depending on the conditions. Over time, established "shrub-oaks" may enlarge in width, being eventually able to shoot up in the centre, where the developing tree becomes inaccessible to herbivores.
Over time, established "shrub-oaks" may enlarge in width, being eventually able to shoot up in the centre, where the developing tree becomes inaccessible to herbivores. Ultimately, pasture-grown oaks, such as these pedunculate oaks in the Breite Oak Tree Reserve near Sighișoara, may form large, old solitaires. In rural landscapes, these old solitary trees are often signs of ancient silvopasture regimes.
Ultimately, pasture-grown oaks, such as these pedunculate oaks in the Breite Oak Tree Reserve near Sighișoara, may form large, old solitaires. In rural landscapes, these old solitary trees are often signs of ancient silvopasture regimes. Eventually, through disease, lightning strike or old age, oaks begin to decay and die, leaving precious habitat for a variety of species.
Eventually, through disease, lightning strike or old age, oaks begin to decay and die, leaving precious habitat for a variety of species.
Ecological anachronisms
Vera prominently argued that since other light-demanding and often thorny woody species exist in Europe—species such as common hawthorn, midland hawthorn, blackthorn, Crataegus rhipidophylla, wild pear and crab apple—their ecology can only be explained under the influence of large herbivores, and that in the absence of these they represent an anachronism.[63][64]
Shortcomings of pollen analysis

Vera further contested that pollen diagrams can adequately display past species occurrences since, inherently, pollen deposits tend to overrepresent species that are wind-pollinated and notoriously underrepresent species that are pollinated by insects.[65] Furthermore, he proposed that an absence of grass pollen in pollen diagrams can be explained by high grazing pressure, which would prevent the grasses from flowering. Under such conditions, he claimed, open environments with only scattered mature trees may appear as closed forests in pollen deposits. He consequently proposed that the conspicuous scarcity of grass pollen in pollen deposits dating from the pre-Neolithic Holocene might not necessarily speak against the existence of open environments dominated by grasses.[66] However, it is generally considered that over 60% tree pollen in pollen deposits indicates a closed forest canopy, which is true for the vast majority of European early to mid-Holocene deposits. Sites with less than 50% arboreal pollen, on the other hand, are consistently associated with human activities.[53]
Circular reasoning
Vera stressed that the prevailing high-forest theory was born out of observations of spontaneous regeneration in the absence of grazing animals. He argued that the presupposition that these animals do not exert a significant influence on natural regeneration, and thus on the vegetation structure as a whole, has been made without comparative confirmation, and is therefore a circular argument. Indeed, modern forestry and forest theory arose largely in the modern era and went hand in hand with the ongoing inclosure of common land throughout Europe. A consequence thereof was in many cases a ban of livestock from the forests, which had previously largely been open woodland pastures, often dominated by oaks. These were multifunctional and used for a range of purposes, from pannage and livestock grazing to the harvest of tree hay, coppice, timber and oak galls for the manufacture of ink, as well as for the production of charcoal, crops and fruit.[67] This former usage of forests is often still revealed by a big age gap between tree generations, particularly if the oldest trees are mainly oaks, and many Central European forest reserves originated as common wood-pastures.
Shifted baselines
In nature conservation, a shifted baseline is a baseline for conservation targets and desired population sizes that is based on non-pristine conditions. In this sense, the term was coined by marine biologist Daniel Pauly when he observed that some fisheries scientists used the population sizes of fish at the beginning of their own careers to assess a desired baseline, notwithstanding whether the fishing stocks they used as baselines had already been diminished by human exploitation. He noticed, that the estimations these scientists took for reference markedly differed from historical accounts. Consequently, he concluded that over generations the perception of what is considered to be normal would change, and so may what is considered a depleted population. Pauly called this the shifting baseline syndrome.[68]

In line with this, it may be argued that the prevalence of closed-canopy forest as the prevailing conservation narrative in Europe similarly arises from multiple shifted baselines:
- While it is plausble that lions (Panthera speleae, P. leo leo), leopards (Panthera pardus spelaea, P. pardus tulliana),[lower-alpha 2] hyenas (Hyaena hyaena prisca, Crocuta crocuta spelaea), dholes (Cuon alpinus europeus), wild ass (Equus hydruntinus) and moon bears (Ursus thibetanus mediterraneus, U. t. permjak), among other victims of European Quaternary and Holocene extinctions, would still be native[72] to Europe, had they not been evicted by humans, none of these species are listed as such in the EU's Habitats Directive's annexes.[73] This absence in conservation law, if applied also to globally extinct megafauna, would imply that elephants and rhinos should be considered native to Europe, too, and hence any landscape that is considered to be natural, yet results from a situation where these are lacking, would necessarily be the consequence of a shifted baseline. It is very likely that the megafauna extinctions of the late Pleistocene and early Holocene had profound implications for European and worldwide ecosystems,[74][75][76] especially given the paramount importance comparable animals have for modern ecosystems.[77][78]
- Vera pointed out that words like wold and forest used to have different connotations than they do today. While today, a forest is a dense and reasonably large tract of trees, the medieval Latin forestis, from which it derives, assigned open stands of trees, and was a wild and uncultivated land home also to aurochs and wild horses. According to historical sources, these forestis included hawthorn, blackthorn, wild cherry, wild apple and wild pear, as well as oaks, all of which are light-demanding species that cannot regenerate successfully in closed-canopy forest. From this Vera concluded that original wildwoods still existed in Europe during the Medieval period. Thus, when scholars of the 19th and 20th century assumed that grazing animals had destroyed the original European closed-canopy wildwoods, they were misinterpreting these terms. Instead, these forests, he found, had been destroyed following the industrial revolution and the population growth it caused, which in turn caused overexploitation.[79]
.jpeg.webp) Many European forests were formerly managed as wood-pasture, coppice or were, as in this case, pollarded.
Many European forests were formerly managed as wood-pasture, coppice or were, as in this case, pollarded. - He further argued that from this initial misinterpretation gave rise to another misinterpretation: that forest regeneration would naturally take place inside the forest. Thus, scholars of the 19th and 20th century such as Elias Landolt (forester) interpreted medieval grazing regulations to allow tree regeneration in coppiced mantle and fringe vegetation as intended to allow regeneration in a forest. In their time, solid firewood was preferred to the medieval coppice bundles, e.g. faggots. However, the production of solid firewood required the felling of trees at an age when they could no longer produce suckers, an ability that trees commonly lose with progressing age. This then led to a different management system: the replacement by saplings planted or naturally regenerated via, for example, shelterwood cuttings. Initially, these trees regenerated inside the forests were differentiated from wild growth outside the forests. In German, the former were referred to as natural regeneration (Naturverjüngung) while the latter had a different name: Holzwildwuchse. Thus, natural regeneration was not synonymous with the natural regeneration of trees in a natural situation. It was not until the 19th and 20th centuries that this distinction was abandoned in German. However, in the absence of thorny nurse bushes, which disappeared due to the shadow under the trees, the planted trees then had to be protected manually. The "natural regeneration" was therefore still depended on work like ploughing, removal of browsing pressure and the suppression of weeds, making it not "natural" in the conventional sense. Instead, according to Vera, the original meaning of the word "natural" in this context was that a seed fell from a tree and then grew by itself, as opposed to being planted. This shift in expectation of where regeneration of trees was to be expected, from thorny fringes of groves in wood-pastures to the interior of closed tree stands, then led to the notion that herbivores were detrimental to forest regeneration, and necessitated fenced-out areas, tree shelters and population control via hunting.[79][80]
- Considered "alien" to the landscape, akin to invasive species, cattle and horses were now also removed from the forests, as it happened in former wood-pastures like Białowieża, because they were seen as harmful to the creation of a new old-growth forest. At the same time, the introduction of the potato made pannage, the fattening of pigs on acorns, obsolete, and grass species specifically bred for a high yield superseded the traditional pasturing, mostly of cattle, in wood-pastures.[79] Together, these mechanisms created the spatial separation between livestock rearing and forestry, grassland and forest enshrined into modern law and practice.
- Finally, the biodiversity losses associated with the conversion of open grassland, mantle and fringe vegetation and open-grown trees into closed-canopy forests were legitimised by the assumption that the forest was the only natural ecosystem, and hence species losses were casualties of a natural cause.[79]
However, a strong argument that may put Vera's etymological evidence into perspective altogether is that the composition of medieval woodlands may not be relevant to their naturalness. Since by the medieval period agricultural traditions had already been ubiquitous in most of Europe for millennia, it may be unrealistic to assume that what people of the time perceived and labelled as wilderness may indeed have been one. Instead, it is doubtful that pristine conditions had survived in the Central- and Western European lowlands, Vera's area of study, at any rate up to this point.[81]
Succession in grazed ecosystems
There are several ecological processes at work in herbivore grazing systems, namely associational resistance, shifting mosaics, cyclic succession, and gap dynamics. These processes would collectively transform the surrounding landscape, as per Vera's model.
Associational resistance
The term associational resistance describes facilitating relationships between plants that grow close to each other, against both biotic and abiotic stresses like browsing, drought, or salinity. In relation to grazed ecosystems, it can allow for the recruitment of trees and other palatable woody species, via thorny nurse bushes, in these environments.[63] It has been proposed and demonstrated that associational resistance can be a key process in grazed environments, ensuring natural succession.[63]
In temperate Europe, succession on pastures commonly starts with so called "islets"[82] ("Geilstellen"), patches of dung which are avoided by the herbivores for an amount of time after deposition, sufficient to allow the establishment of relatively unpalatable species such as rushes, nettles and hummocks of tall grasses like tussock grass.[83] These swards, in turn, provide protection for thorny shrubs such as blackthorn, roses, hawthorn, juniper, bramble, holly and barberry during their early years, when they do not yet have protective thorns and are therefore vulnerable.[84] Once the thorny saplings are fully established, they grow bigger over time and subsequently allow other, less resilient species to establish in their thorn protection, forming mantle and fringe vegetation together with species such as guelder rose, wild privet and dogwood.[65] Other species such as mazzard, checker tree, rowan and whitebeam, which are distributed by fruit-eating birds through their faeces, would also frequently be placed within these shrubs, through resting birds leaving their droppings.[85]
On the other hand, nut-bearing species such as hazel, beech, chestnut, pedunculate and sessile oak would become "planted" somewhat deliberately in the vicinity of those shrubs by rodents such as red squirrel and wood mouse, the nuthatch and corvids such as crows, magpies, ravens and especially jays, which store them for winter supply. In Europe, the Eurasian jay represents the most important seed disperser of oak, burying acorns individually or in small groups. Eurasian jays not only bury acorns in depths favoured by oak saplings, but seemingly also prefer spots with sufficient light availability, i.e. open grassland and transitions between grassland and shrubland, seeking for vertical structures such as shrubs in the near surroundings.[86] Since oak is relatively light-demanding while not having the ability to regenerate on its own under high browsing pressure, these habits of the jay presumably benefit oak, since they provide the conditions oak requires for optimal growth and health.[87] On a similar note, the nuthatch seems to assume a prominent role for hazel dispersal.[88]
In addition, species such as wild pear, crab apple and whitty pear, which bear relatively large fruit, would find propagators in herbivores such as roe deer, red deer and cattle, or in omnivores such as the wild boar, red fox, the European badger and the raccoon,[89] while wind-dispersed species such as maple, elm, lime or ash would land within these shrubs by chance.[90]
Thorny bushes play an important role in tree regeneration in the European lowlands,[91] and evidence is emerging that similar processes can also ensure the survival of browsing-sensitive species like rowan in browsed boreal forests.[92]
Shifting mosaics and cyclic succession

A natural pasture ecosystem would therefore undergo various stages of succession, starting with unpalatable perennial plants, which provide shelter for thorny woody plants. Second, these would start to form thickets and enable the establishment of larger, palatable shrubs and trees respectively. Over time these would then outshadow the unpalatable but light-demanding thickets and emerge as big solitary trees, in the case of single-standing shrubs like hawthorn, or groups of trees in the case of expanding blackthorn shrubs. Because of the herbivore disturbance (browsing, trampling, wallowing, dust bathing), not even shade-tolerant tree saplings would be able to grow under the established trees. Therefore, once the established trees would start to decay, either due to old age or other factors like pathogens, illness, lightning strike or windbreak, this would leave open, bare land behind, for grasses and unpalatable species to colonise, closing the cycle.[93]
On a large scale, different successional stages would thus contribute an ecosystem where open grassland, scrubland, emerging tree growth, groves of trees and solitary trees exist next to each other, and the alternation between these various successional stages would create dynamic shifting mosaics of vegetation.[93] This in turn stimulates high biodiversity.[94][95] Consequently, Vera's counter-proposal to the linear succession and Watt's gap-phase model[12] of closed-canopy forest, to which it has been compared[2] is a model of successional cycles known as the shifting mosaics model.[93]
In effect however, not all areas would have necessarily been subject to this permanent change. Since grazing animals generally prefer to spend time in grasslands rather than in closed stands of trees, it would practically be possible for three different landscape types to coexist over longer periods in the same spots: permanently open areas, permanently closed groves and areas subject to constant shifting mosaics.[96]
The prehistoric baseline
The Eemian landscape
Although Vera himself limited his argument to the Holocene and the fauna present into historical times, research better supports his claims in regard to earlier interglacials.[3][26] Modern humans have likely exerted a strong influence in Europe since their first appearance here during the Weichselian glaciation, which has led some researchers to critizise Vera's choice of the early to mid Holocene as his benchmark for pristine nature. Instead, they argue that pristine nature only existed in Europe before the entering of Homo sapiens.[26] They argue that the best model for what a truly natural landscape during a warm period in Europe would look like is the Eemian interglacial, which was the last warm period before the current Holocene, approximately 130,000 to 115,000 years ago, and the last warm period before Homo sapiens.[lower-alpha 3] While archaic humans existed in the form of neanderthals, their influence was probably only localised, due to their low population density.[98] During this warm period, paleoecological data indeed suggest that semi-open landscapes, as postulated by Vera, were widespread and common, most likely maintained by large herbivores.[26] Next to these semi-open landscapes, however, the researchers also found evidence for closed-canopy forest. Overall, the Eemian landscape appears to have been very dynamic and probably consisted of varying degrees of openness, including open grasslands, wood pastures, light-open woodland and closed-canopy forest.[26]
The European megafauna

The Eemian interglacial was one of many warm interglacials during the Quaternary, of which the Holocene (or Flandrian interglacial) is the most recent. These alternating glacial and interglacial periods, triggered by the Milankovitch cycles, in turn had a profound influence on life. In Middle to Late Pleistocene Europe, the result of this cycling was that two very different faunal and floral assemblages took turns in Central Europe. The warm-temperate Palaeoloxodon-faunal assemblage, consisting of the straight-tusked elephant, Merck's rhinoceros, the narrow-nosed rhinoceros, Hippopotamuses, European water buffalo, aurochs, and several species of deer, among others (including most of today's European fauna), had its core area in the Mediterranean. The warm-temperate assemblage periodically expanded from there into the rest of Europe during warm interglacials, and receded during glacial periods into refugia in the Mediterranean. Meanwhile, the cold-temperate faunal assemblage of the mammoth steppe, consisting of the woolly mammoth, woolly rhinoceros, reindeer, saiga, muskox, steppe bison, arctic fox and lemming among others, was spread across vast areas of Northern Eurasia as well as North America, and during periodic cold glacials advanced deep into Europe. Other animals, such as horses, steppe lions, the scimitar cat, the Ice Age spotted hyena and wolves were part of both faunal assemblages.[99] Both groups of animals spread and retreated cyclically, depending on whether the climate favoured one or the other, but essentially remained intact in refugia that continued to provide the conditions they preferred.
The Quaternary extinction event

Prior to the Last Glacial Maximum however, elements of the warm-temperate Palaeoloxodon-fauna (hippopotamus, straight-tusked elephant, the two Stephanorhinus species and neanderthals, for example) as well as the steppe species Elasmotherium sibricum started to disappear and eventually went extinct. At the onset of the Last Glacial Maximum, populations of Ice Age spotted hyena and the cave bear complex (Ursus spelaea, Ursus ingressus) seem to have collapsed large-scale, and became extinct next. After the Last Glacial Maximum and towards the Holocene, extinctions continued, with many emblematic "Ice Age species" of the mammoth steppe and adjacent habitats, such as the woolly rhinoceros, the steppe lion, the giant deer and the woolly mammoth falling victim, although small regional populations of woolly mammoth and steppe bison held out well into the Holocene,[100][101][102] and the giant deer was present in the southern Ural region into historical times.[103][104] These extinctions have been variously credited to human impact, climate change, or a combination of the two.[105]

These extinctions were not limited to Europe or the Palearctic, but rather occurred on all continents except for Antarctica, in temporal connection to the migration of Homo sapiens. Together, these extinctions are commonly known as the Quaternary extinction event. Whereas today megafaunal Proboscideans, Rhinocerotidae and Hippopotamidae that commonly attain weights of 1,000 kilograms (2,200 lb) exclusively exist in the global south, notably Sub-Saharan Africa and South and Southeast Asia, land mammals of comparable or greater size used to roam the northern hemisphere and South America until relatively recently.[lower-alpha 4] By 10,000 BC, the megafauna of the global north had alternately died out or been severely geographically restricted. Notable examples include various Proboscideans, Rhinocerotidae, ground sloths as well as all South American ungulates, glyptodontines and diprotodontids.
In addition, many mammals above 45 kilograms (99 lb) that were spread across all continents except for Antarctica prior to the Quaternary extinction event have since declined across their range, or become locally or globally extinct, respectively. Modern taxa with a once wider distribution include the Eurasian saiga, wapiti-deer, the Asian black bear, bisons, the dhole, lions, the leopard, the jaguar, and the giant anteater. Research has also shown that the extant megafaunal species that survived the extinction event experienced a sharp population decline starting at the same time and continuing to the present day.[106] While the exact cause of these events remains debated, it seems clear that ecological niches in Europe, the Middle East, big parts of Asia, and the Americas were left unoccupied.
The impact of megafauna extinctions
The effects of the global extinction of megafauna are likely to have been far-reaching and damaging to ecosystems, and continue to be. The late Quaternary extinction event is unprecedented in the Cenozoic (i.e. since the extinction of the non-avian dinosaurs) in its selectivity for large animals.[107][108] Accordingly, the modern European megafauna-extirpated ecosystems deviate strongly from the megafauna-rich evolutionary norm.[109] Similar to how herds of herbivores like wildebeest, zebra, impala, buffalo, and elephants drive African savanna vegetation patterns, and not vice versa (i.e. the vegetation dictates the activities of these herbivores),[110][111][112] it now seems likely that herbivore herds could have provided similar ecosystem functions in the temperate regions before the Quaternary extinctions.[3][26]
In Europe, where many species such as the straight-tusked elephant, two species of Stephanorhinus and the hippopotamus among many others were lost, this meant that their ecosystem functions – such as plant matter consumption and seed dispersal – were lost as well.[109] Without the disturbance these animals provide, it is argued, forests could develop unhindered and landscapes became more uniform.[26][75][113] As this is detrimental to species adapted to the presence of megafauna, some scholars advocate for the reintroduction of these animals where possible, or the introduction of modern proxy species to replace extinct species and their ecological impact, an advocacy known as Pleistocene rewilding.[73]
Towards a resolution
Vera's ideas have been called a "challenge to orthodox thinking"[114] and his book has been widely acclaimed by colleagues.[23] It is credited as the spark of much debate about the character of historic and prehistoric landscapes in Europe.[114][115] However, testing using pollen data generally does not support Vera's claims for widespread semi-open savanna during early stages of the Holocene, but rather lends support to the competing and more widely accepted high-forest theory.[53] Similarly, modelling approaches[116] and the use of beetle diversity as an indicator for landscape openness[3] also support the view of a predominance of forest throughout the early and middle Holocene in most of Europe. Consequently, the botanist John Birks has argued for the rejection of the wood-pasture hypothesis. He did, however, acknowledge that the role grazing animals played in forest composition is being reevaluated, and was formerly largely ignored by Quaternary paleoecologists.[2]
On the other hand, consensus is building that while forest did most likely dominate throughout the early stages of the Holocene, it was never as dense and overarching as previously assumed.[3] Studies also indicate that forest cover varied considerably between regions, and was comparably high in Central Europe and lower in the Atlantic regions.[117] Besides climate, topography must have also played a significant role. The aurochs at least seems to have favoured fertile, low-lying riverine areas and plains,[118] which may have led to locally open conditions, while the hill and mountain ranges were more heavily forested.[20] Overall, dense closed-canopy forest probably covered no more than 60% of most areas, with the remainder divided between open woodlands, savannas and open areas. This made the early to mid-Holocene Europe more forested than either today or during earlier interglacials, but not a continuous woodland.[3]
In a 2005 response to Vera, Kathy Hodder et al. highlighted the importance of disturbance factors other than herbivory, particularly fire, to prehistoric landscapes, pointing out that both the high-forest theory and Vera's model have largely ignored this possibility. This stands in connection to the discovery of fire-loving beetle species and charcoal deposits in the European pre-Neolithic Holocene.[5] In the same paper, they also argued that the influence of large herbivores can be acknowledged without this necessarily implying that they created the open, park-like landscapes described by Vera.[119]
At the same time, modern research has shown that under the current climate free-roaming large grazers can indeed influence and even temporarily halt vegetation succession, as proposed by Vera.[120][121] Vera's choice of the Mesolithic as his benchmark for pristine nature has also been critizised, because the role people played during this period is unclear.[122][123] Anatomically modern humans have been present in Europe since 50-40 kya,[124] and studies indicate that already in the early Holocene, human impact on the environment was second in importance only to climate, surpassing herbivore disturbance.[125] However, the late-Pleistocene expansion of modern humans out of Africa is frequently cited as cause for the simultaneous global extinction of primarily large mammals.[126][127] In a 2014 paper, rewilding ecologist Christopher Sandom et al. found that the depauperate megafauna that remained in Europe after these extinctions may be the reason for the reduced landscape openness. They reached this conclusion by comparing beetle deposits from the Holocene and Eemian of Britain as indicators for the degree of openness. These beetles, they found, indicated that during the Eemian interglacial, the last interglacial with a pristine megafauna, landscape openness was associated with high megafauna densities. In contrast, closed forest predominated in the early Holocene in the absence of megafauna.[3] The importance of the impact of large herbivores on vegetation and the significance of megafauna extinctions in this regard has also been highlighted in other studies.[128][129][38]
Implications and tangents
Implications for conservation practice
Vera's hypothesis has important implications for conservation theory and practice, because it puts emphasis on the importance of grasslands in temperate Europe and their legitimacy as natural landscapes with intrinsic conservation value. Under the high forest framework, these and related landscape types such as heathland were viewed as purely or mostly anthropogenic landscapes, naturally confined to areas marginal enough to prevent woodland formation. Instead it was believed that the broadleaved regions were dominated by climax communities of shade-tolerant species, interrupted only occasionally by collapses of forest cover and disturbances through fire, storm or browsing. Examples of this school of thought include Białowieża on the Polish-Belarusian border as well as the Hainich in Central Germany.

The logical consequence of this was that species associated with grasslands, forest fringes and old, open-grown trees disappeared on large scale, since many ecosystems in Europe, including highly species-rich grasslands in Romania, strictly depend on some management and are negatively impacted if the areas are left fallow and overgrown by forest vegetation.[130][131] Similarly, the displacement of aspen in boreal forests seems to be accelerated more because of increasing competition in the increasingly closed stands than via browsing.[132]
In Europe, grasslands were maintained by large herbivores over the last 1,8 million years,[133] resulting in an exceptional diversity of species in many European grasslands.[133] For example, on a wooded meadow in Estonia, 76 species of plant per 1 square metre (11 sq ft) were counted in 2000, making it one of the world's record sites.[133] Similarly high numbers were counted at other locations in Eastern Europe, making the region one of the hotspots for plant species richness on small scale worldwide.[134] However, grasslands in Europe and elsewhere are increasingly under threat, including from forest encroachment following abandonment, ill-conceived forest restoration schemes, overgrazing and agricultural intensification.[135][136] Especially the notion that most grasslands derive from human management and as such are essentially degraded former woodlands suitable for reforestation[137] has been called into question more recently and is threatening native grassland ecosystems worldwide.[138][139] For Europe, studies have demonstrated the local persistence of grasslands throughout the Holocene as natural ecosystems,[131][51] the important role they play for insects, for example,[140][141] and the potential for biodiversity enhancement that lies in their maintenance by reintroduced large herbivores.[142][143][144] At the same time, up to 90% of European semi-natural grasslands, meaning grasslands that were formerly maintained by humans and their livestock, have disappeared during the 20th century, with losses especially high in Western, Northern and Central Europe.[145]

Given the significant importance oaks have as habitat for wood-eating insect communities in Europe, it has been pointed out that traditional forest management may not deliver all the benefits dead oak wood has for these species, since these often depend on surrounding circumstances such as sun-exposure.[146] Instead, conservation of highly species-rich plant communities of open oak woodlands may best be achieved through traditional grazing management.[147]
In the traditional framework of closed-canopy forest as the aspired ideal, the losses of species dependent on open areas were seen as collateral damage necessary for the creation of this ideal and had to be accepted because species associated with open areas were seen as hemerophiles anyway, which would have followed human clearings into Central and Western Europe only in the Holocene and would have originally been restricted to Southern and Eastern Europe. Taking into account that this results in overall biodiversity loss, traditional agricultural landscapes were then in turn recognised as important refuges for species-groups associated with open landscapes, seen as either a by-product of post-Neolithic agricultural traditions or relics of Pleistocene assemblages that formed alongside the now-extinct Pleistocene megafauna for which introduced domestic animals were partial substitutes.[148] In both cases, their continued survival would largely depend on the continued execution of traditional agricultural practices.
Vera's hypothesis implies both that the model of primeval forest and the resulting rhetoric are the result of a major fallacy in nature conservation, paleoecology and forestry, and that the preservation of open and half-open landscapes and their germane biodiversity does not depend on agricultural practices, but rather on the maintenance by large herbivores, whether wild or domesticated.[149]
Rewilding and practical implementation
The validity of Vera's hypothesis remains debated among ecologists and conservationists, but it is often considered a fruitful approach for conservation, and thus has been widely implemented in daily practice. The resulting rewilding-advocacy differs from more traditional conservation primarily in that it emphasises a hands-off approach. Instead of intervening to preserve or revive specific species or ecosystem types, the principle is to reduce human intervention to a minimum and instead reintroduce natural ecosystem dynamics, with emphasis being put on returning large mammals to the landscape.[150][151][152]

Examples of such projects include the Dutch conservation area Oostvaardersplassen, which was initiated by Vera, as well as the Knepp estate in Sussex. Isabella Tree, co-owner of the latter, has named Vera and his ideas as important reasons for her and her husband to consider rewilding their private estate with fallow deer, red deer, English Longhorn cattle (as ecological proxies for the extinct aurochs) and Tamworth pigs (as proxies for the wild boar).[153]
Furthermore, in the shape of Rewilding Europe, a pan-European organization that aims for creating wild spaces in Europe by re-establishing food chains and reintroducing missing species has identified Vera's proposals as key to complex, biodiverse ecosystems. Taking them into account, it works to establish free-moving herds of European bison, aurochs-proxies (e.g. Taurus-cattle), proxies for the wild tarpan (e.g. Konik, Exmoor pony) as well as water buffalo and kulan (which were present in Europe until the early Holocene) to create dynamic ecosystems maintained by the grazing and browsing activity of these herbivores.[154][155]
Ecology of wood-pastures

Grazed woodlands, wood-pastures and pastures in Europe harbour high biodiversity. Rare perennial plant species commonly or exclusively associated with these ecosystems in Europe include hellebores, peonies, asphodels, dittany, black false hellebore and bastard balm. The tree layer is often dominated by a number of oak species and many rare, local and threatened species such as Florentine wild apple, Lebanese wild apple, medlar, sorb tree, pears and wild plums are more often found in European silvopastoral systems than in commercial forest.[156] Rare or declining bird species such as the European roller, hoopoe, several species of shrike, owls (scops owl, little owl) as well as wrynecks and middle spotted woodpeckers are attracted by wood-pastures in particular. In Iberia, the semi-natural oak-woodlands known as dehesa/montado are home to endemic species such as the Spanish imperial eagle and the Iberian lynx.[157] Wood-pastures also provide important habitat for many species of invertebrates. Due to the abundance of large, old trees, wood-pastures are especially important for saproxylic beetles. This includes spectacular and rare species such as capricorn beetle, stag beetles (such as Lucanus cervus), variable chafer and click beetles. In the British Isles alone nearly 1800 species of invertebrates depend on decaying wood, including 700 species of beetles and about 730 species of flies.[158]
Traditional land use

Many aspects of Vera's theory resonate well with traditional pastoral systems and agricultural practices across Europe and other parts of the world. This is especially true for regions where the pasturing of grazing animals has been carried out for hundreds and thousands of years. The old English saying "The thorn is the mother of the oak", referring to the recruitment of oaks inside thorny shrubs, attests to the knowledge about processes such as associational resistance as part of old traditional farming knowledge that was present in rural communities well before the theory itself was proposed in its current form. The phrase is commonly attributed to Humphry Repton, but was used by the writer Arthur Standish as early as 1613 and probably has origins even earlier.[48] Following Vera's argumentation, wood-pastures and related farming systems as ancient land-use systems can also be viewed as essentially mimicking the primaeval European wilderness. This goes hand-in-hand with the fact that, for instance, 63 of the ecosystems listed in Annex I of the Habitats Directive of the European Union strictly depend on low-intensity use and maintenance work, mostly in the form of grazing and mowing.[67] These habitats are labelled as high nature value farmland (HNV farmland), and the fact that traditional farming, in particular, can potentially harbour exceptional biodiversity values may in part be due to such mimicking effects that some forms of human use (such as grazing, pollarding, coppicing and hedgelaying) have in analogy to ecosystem services formerly exercised by the megafauna.[47]
Sergey Zimov's megaherbivore decline model

While Vera's hypothesis focuses on temperate regions and especially temperate Europe, an argumentatively related model has more recently been proposed for high latitude regions of modern taiga and tundra biomes, where formerly mammoth steppe predominated. It essentially challenges the widespread view that the Pleistocene megafauna of the northern steppe vanished as a consequence of the warming climate at the advent of the Holocene and the consequent turnover of cold-adapted grassland and herb ecosystems into expanding forests and tundra dominated by mosses, lichens and dwarf trees.[159] Instead, it argues that vice versa the declining megafauna was the precondition for the vegetational turnover, and that healthy megafauna populations could have maintained their preferred environment, the mammoth steppe, even under the stresses of the warming climate if human-induced extinctions had not occurred.[160] Consequently, Sergey Zimov, one of the main supporters of this model, proposes that ecosystems functionally similar to the mammoth steppe of the Pleistocene could also function under modern circumstances, and seeks to prove this in the form of Pleistocene park. He and his son have since begun to reintroduce species that are now extinct in Yakutia, and to introduce species that are ecologically similar to those present in the region during the Pleistocene that have since become globally extinct. These include wild species like reindeer, muskox, bison and wisent, as well as hardy domestic breeds like Bactrian camels, Kalmyk cattle, domestic yaks and Orenburg goats.[161] With these, the project hopes to revive the mammoth steppe, at least in fractions of its former expanse.[162]
See also
- New Forest – An ancient common with a significant proportion of wood pasture
- Moccas Park
- Hatfield Forest
- Windsor Great Park
- Epping Forest
- Zuid-Kennemerlan National Park
- Marcescence – possibly an adaptation to prevent browsing of twigs and buds in winter
- Globally Important Agricultural Heritage Systems
Notes
- ↑ The European fallow deer, a mixed feeder and perhaps the closest of the remaining large wild herbivores to a true grazer in Europe,[41] is not usually considered native to temperate Europe, although closely related forms were widespread here in the past, especially during warm interglacial periods. Rather, the modern species has been introduced to much of Europe since antiquity.
- ↑ A small population of leopards survives in the European Caucasus,[69] but there are historical records of Anatolian leopards crossing the Mycale Strait to Greece in the late 1800s.[70] Leopards may have historically persisted elsewhere in Europe, but this is only tentative. The most recent remains of probably wild specimens come from the Balkans and the Iberian Peninsula and date from the early Holocene. Later finds probably come from imported animals.[71]
- ↑ There is evidence for a limited and – for the time being – unsuccesseful human dispersal into Europe more than 200 kya,[97] but neanderthals were still dominant.
- ↑ Bovines are now the only animals outside Africa and Asia that may attain comparable sizes.
References
- ↑ Vera 2000, p. 8.
- 1 2 3 4 Birks, H. John B. (2005-04-01). "Mind the gap: how open were European primeval forests?". Trends in Ecology & Evolution. 20 (4): 154–156. doi:10.1016/j.tree.2005.02.001. ISSN 0169-5347. PMID 16701360.
- 1 2 3 4 5 6 7 8 9 Sandom, Christopher J.; Ejrnæs, Rasmus; Hansen, Morten D. D.; Svenning, Jens-Christian (2014-03-18). "High herbivore density associated with vegetation diversity in interglacial ecosystems". Proceedings of the National Academy of Sciences. 111 (11): 4162–4167. Bibcode:2014PNAS..111.4162S. doi:10.1073/pnas.1311014111. ISSN 0027-8424. PMC 3964052. PMID 24591633.
- 1 2 Rotherham, Ian D., ed. (2013). Trees, Forested Landscapes and Grazing Animals: A European Perspective on Woodlands and Grazed Treescapes. Routledge. ISBN 978-1-138-30448-2.
- 1 2 Buckland, P. C. (2005). "Palaeoecological evidence for the Vera hypothesis?". In Hodder, K.H.; Bullock, J. M.; Buckland, P. C.; Kirby, K. J. (eds.). Large herbivores in the wildwood and modern naturalistic grazing systems. English Nature.
- 1 2 Sugita, Shinya (February 2007). "Theory of quantitative reconstruction of vegetation I: pollen from large sites REVEALS regional vegetation composition". The Holocene. 17 (2): 229–241. Bibcode:2007Holoc..17..229S. doi:10.1177/0959683607075837. ISSN 0959-6836. S2CID 128674351.
- ↑ Vera 2000, p. 11.
- ↑ Vera 2000, p. 30-34.
- ↑ Cotta, Heinrich (1817). Anweisungen zum Waldbau. Dresden.
- ↑ Clements, Frederic E. (Frederic Edward) (1916). Plant succession; an analysis of the development of vegetation. Cornell University Library. Washington, Carnegie Institution of Washington.
- 1 2 Tansley, A. G. (1935). "The Use and Abuse of Vegetational Concepts and Terms". Ecology. 16 (3): 284–307. Bibcode:1935Ecol...16..284T. doi:10.2307/1930070. JSTOR 1930070.
- 1 2 Watt, Alex S. (1947). "Pattern and Process in the Plant Community". Journal of Ecology. 35 (1/2): 1–22. Bibcode:1947JEcol..35....1W. doi:10.2307/2256497. ISSN 0022-0477. JSTOR 2256497.
- ↑ Whittaker, R. H. (1953). "A Consideration of Climax Theory: The Climax as a Population and Pattern". Ecological Monographs. 23 (1): 41–78. Bibcode:1953EcoM...23...41W. doi:10.2307/1943519. ISSN 0012-9615. JSTOR 1943519.
- ↑ Ellenberg, Heinz (1996). Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht (in German) (1st ed.). Stuttgart: Ulmer. ISBN 3-8252-8104-3.
- 1 2 Iversen, Johs (1973-12-31). "Geology of Denmark III: The Development of Denmark's Nature since the Last Glacial". Danmarks Geologiske Undersøgelse V. Række. 7: 1–126. doi:10.34194/raekke5.v7.7020. ISSN 2597-3037.
- 1 2 Firbas, Franz (1949). Spät- und nacheiszeitliche Waldgeschichte Mitteleuropas nördlich der Alpen (in German). Jena: G. Fischer.
- ↑ Prentice, Colin (1988), Huntley, B.; Webb, T. (eds.), "Records of vegetation in time and space: The principles of pollen analysis", Vegetation history, Handbook of vegetation science, Dordrecht: Springer Netherlands, pp. 17–42, doi:10.1007/978-94-009-3081-0_2, ISBN 978-94-009-3081-0, retrieved 2023-11-17
- ↑ Bohn, Udo; Gollub, Gisela (2006). "The Use and Application of the Map of the Natural Vegetation of Europe with Particular Reference to Germany". Biology and Environment: Proceedings of the Royal Irish Academy. 106B (3): 199–213. doi:10.1353/bae.2006.0002. ISSN 2009-003X.
- ↑ Nielsen, Anne Birgitte; Giesecke, Thomas; Theuerkauf, Martin; Feeser, Ingo; Behre, Karl-Ernst; Beug, Hans-Jürgen; Chen, Su-Hwa; Christiansen, Jörg; Dörfler, Walter; Endtmann, Elisabeth; Jahns, Susanne (2012-07-30). "Quantitative reconstructions of changes in regional openness in north-central Europe reveal new insights into old questions". Quaternary Science Reviews. 47: 131–149. Bibcode:2012QSRv...47..131N. doi:10.1016/j.quascirev.2012.05.011. ISSN 0277-3791.
- 1 2 3 4 Svenning, Jens-Christian (2002-04-01). "A review of natural vegetation openness in north-western Europe". Biological Conservation. 104 (2): 133–148. Bibcode:2002BCons.104..133S. doi:10.1016/S0006-3207(01)00162-8. ISSN 0006-3207.
- ↑ Kuneš, Petr; Svobodová-Svitavská, Helena; Kolář, Jan; Hajnalová, Mária; Abraham, Vojtěch; Macek, Martin; Tkáč, Peter; Szabó, Péter (2015-05-15). "The origin of grasslands in the temperate forest zone of east-central Europe: long-term legacy of climate and human impact". Quaternary Science Reviews. 116: 15–27. Bibcode:2015QSRv..116...15K. doi:10.1016/j.quascirev.2015.03.014. ISSN 0277-3791. PMC 5433559. PMID 28522887.
- ↑ Birks, H. J. B. "Past forests of Europe" (PDF). Retrieved 2022-06-19.
- 1 2 Vera, F. W. M., ed. (January 2000). Grazing ecology and forest history (1 ed.). UK: CABI Publishing. doi:10.1079/9780851994420.0000. ISBN 978-0-85199-442-0.
- ↑ Gaillard, M.-J.; Sugita, S.; Mazier, F.; Trondman, A.-K.; Broström, A.; Hickler, T.; Kaplan, J. O.; Kjellström, E.; Kokfelt, U.; Kuneš, P.; Lemmen, C.; Miller, P.; Olofsson, J.; Poska, A.; Rundgren, M. (2010-07-26). "Holocene land-cover reconstructions for studies on land cover-climate feedbacks". Climate of the Past. 6 (4): 483–499. Bibcode:2010CliPa...6..483G. doi:10.5194/cp-6-483-2010. ISSN 1814-9324.
- ↑ Githumbi, Esther; Fyfe, Ralph; Gaillard, Marie-Jose; Trondman, Anna-Kari; Mazier, Florence; Nielsen, Anne-Birgitte; Poska, Anneli; Sugita, Shinya; Woodbridge, Jessie; Azuara, Julien; Feurdean, Angelica; Grindean, Roxana; Lebreton, Vincent; Marquer, Laurent; Nebout-Combourieu, Nathalie (2022-04-08). "European pollen-based REVEALS land-cover reconstructions for the Holocene: methodology, mapping and potentials". Earth System Science Data. 14 (4): 1581–1619. Bibcode:2022ESSD...14.1581G. doi:10.5194/essd-14-1581-2022. hdl:11380/1274659. ISSN 1866-3508.
- 1 2 3 4 5 6 7 8 9 Pearce, Elena A.; Mazier, Florence; Normand, Signe; Fyfe, Ralph; Andrieu, Valérie; Bakels, Corrie; Balwierz, Zofia; Bińka, Krzysztof; Boreham, Steve; Borisova, Olga K.; Brostrom, Anna; de Beaulieu, Jacques-Louis; Gao, Cunhai; González-Sampériz, Penélope; Granoszewski, Wojciech (2023-11-10). "Substantial light woodland and open vegetation characterized the temperate forest biome before Homo sapiens". Science Advances. 9 (45): eadi9135. Bibcode:2023SciA....9I9135P. doi:10.1126/sciadv.adi9135. ISSN 2375-2548. PMC 10637746. PMID 37948521.
- ↑ "Strategy for conservation of the european bison". WWF Russia. Retrieved 2022-06-19.
- ↑ Munro, Paul. "Deer numbers placing unprecedented pressure on environment". Forestry and Land Scotland. Retrieved 2022-06-19.
- ↑ Warren, R. J. (2011-04-08). "Deer overabundance in the USA: recent advances in population control". Animal Production Science. 51 (4): 259–266. doi:10.1071/AN10214. ISSN 1836-5787.
- ↑ "Why manage Deer?". The British Deer Society. Retrieved 2022-06-19.
- ↑ Tanentzap, Andrew J.; Kirby, Keith J.; Goldberg, Emma (2012-01-15). "Slow responses of ecosystems to reductions in deer (Cervidae) populations and strategies for achieving recovery". Forest Ecology and Management. 264: 159–166. doi:10.1016/j.foreco.2011.10.005. ISSN 0378-1127.
- ↑ Hanberry, Brice B.; Faison, Edward K. (2023-04-10). "Re-framing deer herbivory as a natural disturbance regime with ecological and socioeconomic outcomes in the eastern United States". Science of the Total Environment. 868: 161669. Bibcode:2023ScTEn.868p1669H. doi:10.1016/j.scitotenv.2023.161669. ISSN 0048-9697. PMID 36681343. S2CID 256079572.
- ↑ Hopcraft, J. Grant C.; Ollf, Han; Sinclair, A. R. E. (2010). "Herbivores, resources and risks: alternating regulation along primary environmental gradients in savannas". Trends in Ecology & Evolution. 25 (2): 119–128. doi:10.1016/j.tree.2009.08.001. PMID 19767121 – via Cell Press.
- ↑ Jędrzejewski, Włodzimierz; Schmidt, Krzysztof; Theuerkauf, Jörn; Jędrzejewska, Bogumiła; Selva, Nuria; Zub, Karol; Szymura, Lucyna (May 2002). "Kill Rates and Predation by Wolves On Ungulate Populations in Białowieża Primeval Forest (Poland)". Ecology. 83 (5): 1341–1356. doi:10.1890/0012-9658(2002)083[1341:KRAPBW]2.0.CO;2. ISSN 0012-9658.
- 1 2 Skogland, Terje (1991). "What Are the Effects of Predators on Large Ungulate Populations?". Oikos. 61 (3): 401–411. Bibcode:1991Oikos..61..401S. doi:10.2307/3545248. ISSN 0030-1299. JSTOR 3545248.
- ↑ Pringle, Robert M. (2018-08-06). "Ecology: Megaherbivores Homogenize the Landscape of Fear". Current Biology. 28 (15): R835–R837. doi:10.1016/j.cub.2018.06.050. ISSN 0960-9822. PMID 30086317. S2CID 51933977.
- ↑ Mduma, Simon A. R.; Sinclair, A. R. E.; Hilborn, Ray (November 1999). "Food regulates the Serengeti wildebeest: a 40-year record". Journal of Animal Ecology. 68 (6): 1101–1122. Bibcode:1999JAnEc..68.1101M. doi:10.1046/j.1365-2656.1999.00352.x. ISSN 0021-8790.
- 1 2 Fløjgaard, Camilla; Pedersen, Pil Birkefeldt Møller; Sandom, Christopher J.; Svenning, Jens-Christian; Ejrnæs, Rasmus (2022). "Exploring a natural baseline for large-herbivore biomass in ecological restoration". Journal of Applied Ecology. 59 (1): 18–24. Bibcode:2022JApEc..59...18F. doi:10.1111/1365-2664.14047. S2CID 212880616 – via Wiley Online Library.
- ↑ Bradshaw, Richard; Mitchell, Fraser J. G (1999-07-12). "The palaeoecological approach to reconstructing former grazing–vegetation interactions". Forest Ecology and Management. 120 (1): 3–12. doi:10.1016/S0378-1127(98)00538-6. ISSN 0378-1127.
- ↑ Searle, Kate R.; Shipley, Lisa A. (2008), Gordon, Iain J.; Prins, Herbert H. T. (eds.), "The Comparative Feeding Bahaviour of Large Browsing and Grazing Herbivores", The Ecology of Browsing and Grazing, Ecological Studies, Berlin, Heidelberg: Springer, vol. 195, pp. 117–148, doi:10.1007/978-3-540-72422-3_5, ISBN 978-3-540-72422-3, retrieved 2022-07-30
- ↑ Spitzer, R; Felton, A; Landmann, M; Singh, N. J.; Widemo, F; Cromsigt J.P.G.M. (6 July 2020). "Fifty years of European ungulate dietary studies: a synthesis". Oikos. 129 (11): 1668–1680. Bibcode:2020Oikos.129.1668S. doi:10.1111/oik.07435.
- ↑ "Tauros". Rewilding Europe. Retrieved 2022-07-29.
- ↑ "Wild horses". Rewilding Europe. Retrieved 2022-07-29.
- ↑ Abrams, Marc D. (1992). "Fire and the Development of Oak Forests". BioScience. 42 (5): 346–353. doi:10.2307/1311781. ISSN 0006-3568. JSTOR 1311781.
- ↑ Whitehouse, Nicki J.; Smith, David (February 2010). "How fragmented was the British Holocene wildwood? Perspectives on the "Vera" grazing debate from the fossil beetle record". Quaternary Science Reviews. 29 (3): 539. Bibcode:2010QSRv...29..539W. doi:10.1016/j.quascirev.2009.10.010. S2CID 128691666 – via Elsevier.
- ↑ Olsson, Fredrik; Lemdahl, Geoffrey (20 August 2009). "A continuous Holocene beetle record from the site Stavsåkra, southern Sweden: implications for the last 10 600 years of forest and land use history†". Journal of Quaternary Science. 24 (6): 612–626. Bibcode:2009JQS....24..612O. doi:10.1002/jqs.1242. S2CID 140559497 – via Wiley Online Library.
- 1 2 Pykälä, Juha (2000). "Mitigating Human Effects on European Biodiversity through Traditional Animal Husbandry". Conservation Biology. 14 (3): 705–712. Bibcode:2000ConBi..14..705P. doi:10.1046/j.1523-1739.2000.99119.x. ISSN 1523-1739. S2CID 53393839.
- 1 2 3 Green, Ted (2013). "Ancient trees and wood-pastures". In Rotherham, Ian D. (ed.). Trees, Forested Landscapes and Grazing Animals: a European Perspective on Woodlands and Grazed Treescapes. Abingdon, Oxon: Routledge. p. 141. ISBN 9781138304482.
- ↑ Schüle, Wilhelm (1992). "Vegetation, Megaherbivores, Man and Climate in the Quaternary and the Genesis of Closed Forests". In Goldammer, Johann Georg (ed.). Tropical Forests in Transition: Ecology of Natural and Anthropogenic Disturbance Processes. Freiburg: Birkhäuser. ISBN 978-3-0348-7256-0.
- ↑ Rackham, Oliver (1998). "Savanna in Europe". In Kirby, K.J.; Watkins, C. (eds.). The Ecological History of European Forests. Wallingford: CAB International.
- 1 2 Hejcman, M.; Hejcmanová, P.; Pavlů, V.; Beneš, J. (2013). "Origin and history of grasslands in Central Europe – a review". Grass and Forage Science. 68 (3): 345. Bibcode:2013GForS..68..345H. doi:10.1111/gfs.12066. ISSN 0142-5242.
- ↑ Bobiec, Andrzej; Jaszcz, Ewelina; Wojtunik, Karolina (2011-09-01). "Oak (Quercus robur L.) regeneration as a response to natural dynamics of stands in European hemiboreal zone". European Journal of Forest Research. 130 (5): 785–797. doi:10.1007/s10342-010-0471-3. ISSN 1612-4677. S2CID 20878128.
- 1 2 3 Mitchell, Fraser (2005). "How open were European primeval forests? Hypothesis testing using palaeoecological data". Journal of Ecology. 93 (1): 168–177. Bibcode:2005JEcol..93..168M. doi:10.1111/j.1365-2745.2004.00964.x. S2CID 85163768.
- ↑ Dietz, Lucie; Gégout, Jean-Claude; Dupouey, Jean-Luc; Lacombe, Eric; Laurent, Lisa; Collet, Catherine (2022-01-01). "Beech and hornbeam dominate oak 20 years after the creation of storm-induced gaps". Forest Ecology and Management. 503: 119758. doi:10.1016/j.foreco.2021.119758. ISSN 0378-1127. S2CID 241017188.
- 1 2 Giesecke, Thomas; Brewer, Simon (2018-03-01). "Notes on the postglacial spread of abundant European tree taxa". Vegetation History and Archaeobotany. 27 (2): 337–349. Bibcode:2018VegHA..27..337G. doi:10.1007/s00334-017-0640-0. ISSN 1617-6278. S2CID 135162790.
- ↑ Jamrichová, E.; Hédl, R.; Kolář, J.; Tóth, P.; Bobek, P.; Hajnalová, M.; Procházka, J.; Kadlec, J.; Szabó, P. (2017-10-01). "Human impact on open temperate woodlands during the middle Holocene in Central Europe". Review of Palaeobotany and Palynology. 245: 55–68. Bibcode:2017RPaPa.245...55J. doi:10.1016/j.revpalbo.2017.06.002. ISSN 0034-6667.
- 1 2 3 Bobiec, Andrzej; Reif, Albert; Öllerer, Kinga (2018-04-01). "Seeing the oakscape beyond the forest: a landscape approach to the oak regeneration in Europe". Landscape Ecology. 33 (4): 513–528. Bibcode:2018LaEco..33..513B. doi:10.1007/s10980-018-0619-y. ISSN 1572-9761. S2CID 4435032.
- ↑ Uytvanck, Jan Van; Maes, Dirk; Vandenhaute, Dominique; Hoffmann, Maurice (2008-01-01). "Restoration of woodpasture on former agricultural land: The importance of safe sites and time gaps before grazing for tree seedlings". Biological Conservation. 141 (1): 78–88. Bibcode:2008BCons.141...78U. doi:10.1016/j.biocon.2007.09.001. hdl:1854/LU-392964. ISSN 0006-3207.
- ↑ Kollmann, Johannes; Schill, Hans-Peter (1996-08-01). "Spatial patterns of dispersal, seed predation and germination during colonization of abandoned grassland by Quercus petraea and Corylus avellana". Vegetatio. 125 (2): 193–205. doi:10.1007/BF00044651. ISSN 1573-5052. S2CID 26495384.
- ↑ Kuiters, A. T; Slim, P. A (2003-08-03). "Tree colonisation of abandoned arable land after 27 years of horse-grazing: the role of bramble as a facilitator of oak wood regeneration". Forest Ecology and Management. Forest Dynamics and Ungulate Herbivory : From Leaf to Landscape. 181 (1): 239–251. doi:10.1016/S0378-1127(03)00136-1. ISSN 0378-1127.
- ↑ Demeter, László; Molnár, Ábel Péter; Öllerer, Kinga; Csóka, György; Kiš, Alen; Vadász, Csaba; Horváth, Ferenc; Molnár, Zsolt (2021-01-01). "Rethinking the natural regeneration failure of pedunculate oak: The pathogen mildew hypothesis". Biological Conservation. 253: 108928. Bibcode:2021BCons.25308928D. doi:10.1016/j.biocon.2020.108928. ISSN 0006-3207. S2CID 232343559.
- ↑ Mougou, A.; Dutech, C.; Desprez-Loustau, M.-L. (2008). "New insights into the identity and origin of the causal agent of oak powdery mildew in Europe". Forest Pathology. 38 (4): 275–287. doi:10.1111/j.1439-0329.2008.00544.x. ISSN 1439-0329.
- 1 2 3 Bakker, E. S.; Olff, H.; Vandenberghe, C.; Maeyer, K. De; Smit, R.; Gleichman, J. M.; Vera, F. W. M. (2004). "Ecological anachronisms in the recruitment of temperate light-demanding tree species in wooded pastures". Journal of Applied Ecology. 41 (3): 571–582. Bibcode:2004JApEc..41..571B. doi:10.1111/j.0021-8901.2004.00908.x. ISSN 1365-2664. S2CID 84841205.
- ↑ Vera, Frans W. M.; Bakker, Elisabeth S.; Olff, Han. "Large herbivores: missing partners of western European light-demanding tree and shrub species?" (PDF).
- 1 2 Vera 2000, p. 86.
- ↑ Vera 2000, p. 87-88.
- 1 2 Oppermann, Rainer (2014). "Wood-pastures as examples of European high nature value landscapes". In Plieninger, Tobias; Hartel, Tobias (eds.). European Wood-pastures in Transition. Routledge.
- ↑ Pauly, Daniel (1995). "Anecdotes and the shifting baseline syndrome of fisheries" (PDF). Trends in Ecology and Evolution. 10 (10): 430. doi:10.1016/S0169-5347(00)89171-5. PMID 21237093.
- ↑ Stein, A.B.; Athreya, V.; Gerngross, P.; Balme, G.; Henschel, P.; Karanth, U.; Miquelle, D.; Rostro-Garcia, S.; Kamler, J. F.; Laguardia, A.; Khorozyan, I. & Ghoddousi, A. (2020) [amended version of 2019 assessment]. "Panthera pardus". IUCN Red List of Threatened Species. 2020: e.T15954A163991139. doi:10.2305/IUCN.UK.2020-1.RLTS.T15954A163991139.en. Retrieved 15 January 2022.
- ↑ Masetti, M. (2012). Atlas of terrestrial mammals of the Ionian and Aegean islands. Walter de Gruyter.
- ↑ Sommer, R. S.; Benecke, N. (2006). "Late Pleistocene and Holocene development of the felid fauna (Felidae) of Europe: a review". Journal of Zoology. 269: 7–19. doi:10.1111/j.1469-7998.2005.00040.x.
- ↑ Lemoine, Rhys T.; Svenning, Jens-Christian (2022-03-03). "Nativeness is not binary—a graduated terminology for native and non-native species in the Anthropocene". Restoration Ecology. 30 (8). Bibcode:2022ResEc..3013636L. doi:10.1111/rec.13636. ISSN 1061-2971. S2CID 246251260.
- 1 2 Trouwborst, Arie; Svenning, Jens-Christian (25 April 2022). "Megafauna restoration as a legal obligation: International biodiversity law and the rehabilitation of large mammals in Europe". Review of European, Comparative & International Environmental Law. 31 (2): 182–198. doi:10.1111/reel.12443.
- ↑ Galetti, Mauro; Moleón, Marcos; Jordano, Pedro; Pires, Mathias M.; Guimarães, Paulo R.; Pape, Thomas; Nichols, Elizabeth; Hansen, Dennis; Olesen, Jens M.; Munk, Michael; de Mattos, Jacqueline S. (May 2018). "Ecological and evolutionary legacy of megafauna extinctions: Anachronisms and megafauna interactions". Biological Reviews. 93 (2): 845–862. doi:10.1111/brv.12374. PMID 28990321. S2CID 4762203.
- 1 2 Rowan, John; Faith, J. T. (2019), Gordon, Iain J.; Prins, Herbert H. T. (eds.), "The Paleoecological Impact of Grazing and Browsing: Consequences of the Late Quaternary Large Herbivore Extinctions", The Ecology of Browsing and Grazing II, Ecological Studies, Cham: Springer International Publishing, vol. 239, pp. 61–79, doi:10.1007/978-3-030-25865-8_3, ISBN 978-3-030-25865-8, S2CID 210622244, retrieved 2021-09-11
- ↑ Berti, Emilio; Svenning, Jens-Christian (December 2020). Lyons, Kathleen (ed.). "Megafauna extinctions have reduced biotic connectivity worldwide". Global Ecology and Biogeography. 29 (12): 2131–2142. Bibcode:2020GloEB..29.2131B. doi:10.1111/geb.13182. ISSN 1466-822X. S2CID 224954067.
- ↑ Owen-Smith, Norman (1992). Megaherbivores: The Influence Of Very Large Body Size On Ecology. Cambridge University Press. ISBN 9780521426374.
- ↑ Owen-Smith, Norman (1987). "Pleistocene extinctions: the pivotal role of megaherbivores". Paleobiology. 13 (3): 351–362. Bibcode:1987Pbio...13..351O. doi:10.1017/S0094837300008927. ISSN 0094-8373. S2CID 83753159.
- 1 2 3 4 red VERA, FRANS (2009). "The Shifting Baseline Syndrome in Restoration Ecology". In Hall, Marcus (ed.). Restoration and History. Routledge. doi:10.4324/9780203860373. ISBN 978-0-203-86037-3.
- ↑ Vera 2000, p. 102-183.
- ↑ Szabó, Péter (2009-05-22). "Open woodland in Europe in the Mesolithic and in the Middle Ages: Can there be a connection?". Forest Ecology and Management. 257 (12): 2327–2330. doi:10.1016/j.foreco.2009.03.035. ISSN 0378-1127.
- ↑ Dittrich, Alex D. K.; Helden, Alvin J. (February 2012). "Experimental sward islets: the effect of dung and fertilisation on Hemiptera and Araneae: Experimental islets and arthropods". Insect Conservation and Diversity. 5 (1): 46–56. doi:10.1111/j.1752-4598.2011.00133.x. S2CID 83922133.
- ↑ Smit, Christian; Ruifrok, Jasper Laurens (June 2011). "From protégé to nurse plant: establishment of thorny shrubs in grazed temperate woodlands: Establishment of thorny nurse shrubs". Journal of Vegetation Science. 22 (3): 377–386. doi:10.1111/j.1654-1103.2011.01264.x.
- ↑ Rackham, Oliver (1980). Ancient woodland, its history, vegetation and uses in England. London, UK: Castlepoint Press. ISBN 978-1897604274.
- ↑ Vera 2000, p. 341-342.
- ↑ den Ouden, Jan; Jansen, Patrick A.; Smit, Ruben (2004-12-20). "Jays, Mice and Oaks: Predation and Dispersal of Quercus robur and Q. petraea in North-western Europe". In Lambert, J. E.; Hulme, P. E.; Vander Wall, S. B. (eds.). Seed Fate: Predation, Dispersal, and Seedling Establishment. CABI. ISBN 978-0-85199-072-9.
- ↑ Vera 2000, p. 300-307.
- ↑ Vera 2000, p. 338-339.
- ↑ Vera 2000, pp. 341–342.
- ↑ Vera 2000, p. 329-333.
- ↑ Salek, Lubomir; Harmacek, Jaromir; Jerabkova, Lucie; Topacoglu, Osman; Machar, Ivo (January 2019). "Thorny Shrubs Limit the Browsing Pressure of Large Herbivores on Tree Regeneration in Temperate Lowland Forested Landscapes". Sustainability. 11 (13): 3578. doi:10.3390/su11133578. ISSN 2071-1050.
- ↑ Hegland, Stein Joar; Rydgren, Knut; Lilleeng, Marte S.; Moe, Stein R.; Gillespie, Mark A. K. (2021-11-15). "Junipers enable heavily browsed rowan saplings to escape ungulates in boreal forest". Forest Ecology and Management. 500: 119651. doi:10.1016/j.foreco.2021.119651. hdl:11250/2986091. ISSN 0378-1127. S2CID 239702131.
- 1 2 3 Olff, H.; Vera, F. W. M.; Bokdam, J.; Bakker, E. S.; Gleichman, J. M.; Maeyer, K. de; Smit, R. (1999). "Shifting Mosaics in Grazed Woodlands Driven by the Alternation of Plant Facilitation and Competition". Plant Biology. 1 (2): 127–137. Bibcode:1999PlBio...1..127O. doi:10.1111/j.1438-8677.1999.tb00236.x. ISSN 1438-8677.
- ↑ Moreno, Gerardo; Gonzalez-Bornay, Guillermo; Pulido, Fernando; Lopez-Diaz, María Lourdes; Bertomeu, Manuel; Juárez, Enrique; Diaz, Mario (2016-02-01). "Exploring the causes of high biodiversity of Iberian dehesas: the importance of wood pastures and marginal habitats". Agroforestry Systems. 90 (1): 87–105. Bibcode:2016AgrSy..90...87M. doi:10.1007/s10457-015-9817-7. ISSN 1572-9680. S2CID 18441263.
- ↑ Oksuz, Duygu P.; Aguiar, Carlos A. S.; Tápia, Susana; Llop, Esteve; Lopes, Paula; Serrano, Artur R. M.; Leal, Ana I.; Branquinho, Cristina; Correia, Otilia; Rainho, Ana; Correia, Ricardo A. (2020-05-15). "Increasing biodiversity in wood-pastures by protecting small shrubby patches". Forest Ecology and Management. 464: 118041. doi:10.1016/j.foreco.2020.118041. hdl:10451/45341. ISSN 0378-1127. S2CID 216294452.
- ↑ Kirby, K. J. (1 September 2004). "A model of a natural wooded landscape in Britain as influenced by large herbivore activity". Forestry: An International Journal of Forest Research. 77 (5): 405–420. doi:10.1093/forestry/77.5.405 – via Oxford Academic.
- ↑ Harvati, K.; Röding, C.; Bosman, A. M.; Karakostis, F. A.; Grün, R.; Stringer, C.; Karkanas, P.; Thompson, N. C.; Koutoulidis, V.; Moulopoulos, L. A.; Gorgoulis, V. G.; Kouloukoussa, M. (10 July 2019). "Apidima Cave fossils provide earliest evidence of Homo sapiens in Eurasia". Nature. 571 (7766): 500–504. doi:10.1038/s41586-019-1376-z. ISSN 1476-4687. PMID 31292546. S2CID 195873640.
- ↑ Pop, Eduard; Bakels, Corrie (2015-06-01). "Semi-open environmental conditions during phases of hominin occupation at the Eemian Interglacial basin site Neumark-Nord 2 and its wider environment". Quaternary Science Reviews. 117: 72–81. Bibcode:2015QSRv..117...72P. doi:10.1016/j.quascirev.2015.03.020. ISSN 0277-3791.
- ↑ Kurtén, Björn (1968). Pleistocene Mammals of Europe. Tayler and Francis. ISBN 0202309533.
- ↑ Zazula, Grant D.; Hall, Elizabeth; Hare, P. Gregory; Thomas, Christian; Mathewes, Rolf; La Farge, Catherine; Martel, André L.; Heintzman, Peter D.; Shapiro, Beth (November 2017). "A middle Holocene steppe bison and paleoenvironments from the Versleuce Meadows, Whitehorse, Yukon, Canada". Canadian Journal of Earth Sciences. 54 (11): 1138–1152. Bibcode:2017CaJES..54.1138Z. doi:10.1139/cjes-2017-0100. hdl:1807/78639. ISSN 0008-4077. S2CID 54951935.
- ↑ Vartanyan, S. L.; Garutt, V. E.; Sher, A. V. (March 1993). "Holocene dwarf mammoths from Wrangel Island in the Siberian Arctic". Nature. 362 (6418): 337–340. Bibcode:1993Natur.362..337V. doi:10.1038/362337a0. ISSN 1476-4687. PMID 29633990. S2CID 4249191.
- ↑ Murchie, Tyler J.; Monteath, Alistair J.; Mahony, Matthew E.; Long, George S.; Cocker, Scott; Sadoway, Tara; Karpinski, Emil; Zazula, Grant; MacPhee, Ross D. E.; Froese, Duane; Poinar, Hendrik N. (2021-12-08). "Collapse of the mammoth-steppe in central Yukon as revealed by ancient environmental DNA". Nature Communications. 12 (1): 7120. Bibcode:2021NatCo..12.7120M. doi:10.1038/s41467-021-27439-6. ISSN 2041-1723. PMC 8654998. PMID 34880234.
- ↑ Van der Pflicht, J. (2015). "New Holocene refugia of giant deer (Megaloceros giganteus Blum.) in Siberia: updated extinction patterns". Quaternary Science Reviews. 114: 182–188. Bibcode:2015QSRv..114..182V. doi:10.1016/j.quascirev.2015.02.013.
- ↑ Stuart, A. J.; Kosintsev, P. A.; Higham, T. F. G.; Lister, A. M. (October 2004). "Pleistocene to Holocene extinction dynamics in giant deer and woolly mammoth". Nature. 431 (7009): 684–689. Bibcode:2004Natur.431..684S. doi:10.1038/nature02890. ISSN 1476-4687. PMID 15470427. S2CID 4415073.
- ↑ Stuart, A.; Lister, A. (2007). "Patterns of Late Quaternary megafaunal extinctions in Europe and northern Asia" (PDF). S2CID 135226876.
- ↑ Bergman, Juraj; Pedersen, Rasmus Ø; Lundgren, Erick J.; Lemoine, Rhys T.; Monsarrat, Sophie; Pearce, Elena A.; Schierup, Mikkel H.; Svenning, Jens-Christian (2023-11-24). "Worldwide Late Pleistocene and Early Holocene population declines in extant megafauna are associated with Homo sapiens expansion rather than climate change". Nature Communications. 14 (1): 7679. Bibcode:2023NatCo..14.7679B. doi:10.1038/s41467-023-43426-5. ISSN 2041-1723. PMC 10667484. PMID 37996436.
- ↑ Smith, Felisa A.; Elliott Smith, Rosemary E.; Lyons, S. Kathleen; Payne, Jonathan L.; Villaseñor, Amelia (2019-05-01). "The accelerating influence of humans on mammalian macroecological patterns over the late Quaternary". Quaternary Science Reviews. 211: 1–16. Bibcode:2019QSRv..211....1S. doi:10.1016/j.quascirev.2019.02.031. ISSN 0277-3791.
- ↑ Smith, Felisa A.; Elliott Smith, Rosemary E.; Lyons, S. Kathleen; Payne, Jonathan L. (2018-04-20). "Body size downgrading of mammals over the late Quaternary". Science. 360 (6386): 310–313. Bibcode:2018Sci...360..310S. doi:10.1126/science.aao5987. ISSN 0036-8075. PMID 29674591. S2CID 5046004.
- 1 2 Davoli, Marco; Monsarrat, Sophie; Pedersen, Rasmus Østergaard; Scussolini, Paolo; Karger, Dirk Nikolaus; Normand, Signe; Svenning, Jens-Christian (2023-11-17). "Megafauna diversity and functional declines in Europe from the Last Interglacial to the present". Global Ecology and Biogeography. 33: 34–47. doi:10.1111/geb.13778. ISSN 1466-822X. S2CID 265314220.
- ↑ Moe, Stein R.; Rutina, Lucas P.; Hytteborn, Håkan; du Toit, Johan T. (14 January 2009). "What controls woodland regeneration after elephants have killed the big trees?". Journal of Applied Ecology. 46 (1): 223–230. Bibcode:2009JApEc..46..223M. doi:10.1111/j.1365-2664.2008.01595.x.
- ↑ O’Kane, Christopher A. J.; Duffy, Kevin J.; Page, Bruce R.; Macdonald, David W. (2011-09-01). "Are the long-term effects of mesobrowsers on woodland dynamics substitutive or additive to those of elephants?". Acta Oecologica. 37 (5): 393–398. Bibcode:2011AcO....37..393O. doi:10.1016/j.actao.2011.05.005. ISSN 1146-609X.
- ↑ Carroll, Sean (2016). The Serengeti Rules. Princeton, New Jersey: Princeton University Press. p. 143. ISBN 978-0-691-17568-3.
- ↑ Johnson, C.N. (2009-07-22). "Ecological consequences of Late Quaternary extinctions of megafauna". Proceedings of the Royal Society B: Biological Sciences. 276 (1667): 2509–2519. doi:10.1098/rspb.2008.1921. ISSN 0962-8452. PMC 2684593. PMID 19324773.
- 1 2 Hodder, Kathryn H.; Buckland, Paul C.; Kirby, Keith K.; Bullock, J. M. (2009). "Can the mid-Holocene provide suitable models for rewilding the landscape in Britain?". British Wildlife. 20 (5): 4–15. ISSN 0958-0956.
- ↑ "The nature of the first forests in North-west Europe". The Old Man of Wytham. 2018-10-28. Retrieved 2022-06-22.
- ↑ Soepboer, W.; Lotter, A. F. (2009-01-01). "Estimating past vegetation openness using pollen–vegetation relationships: A modelling approach". Review of Palaeobotany and Palynology. 153 (1): 102–107. Bibcode:2009RPaPa.153..102S. doi:10.1016/j.revpalbo.2008.07.004. ISSN 0034-6667.
- ↑ Roberts, N.; Fyfe, R. M.; Woodbridge, J.; Gaillard, M.-J.; Davis, B. a. S.; Kaplan, J. O.; Marquer, L.; Mazier, F.; Nielsen, A. B.; Sugita, S.; Trondman, A.-K. (2018-01-15). "Europe's lost forests: a pollen-based synthesis for the last 11,000 years". Scientific Reports. 8 (1): 716. Bibcode:2018NatSR...8..716R. doi:10.1038/s41598-017-18646-7. ISSN 2045-2322. PMC 5768782. PMID 29335417.
- ↑ Hall, Stephen J. G. (April 2008). "A comparative analysis of the habitat of the extinct aurochs and other prehistoric mammals in Britain". Ecography. 31 (2): 187–190. Bibcode:2008Ecogr..31..187H. doi:10.1111/j.0906-7590.2008.5193.x. ISSN 0906-7590.
- ↑ Hodder, Kathy H.; Bullock, J.M.; Buckland, Paul C.; Kirby, K.J. (2005). Large herbivores in the wildwood and modern naturalistic grazing systems. English Nature. p. 52.
- ↑ Cornelissen, Perry; Bokdam, Jan; Sykora, Karlè; Berendse, Frank (2014-08-01). "Effects of large herbivores on wood pasture dynamics in a European wetland system". Basic and Applied Ecology. 15 (5): 396–406. doi:10.1016/j.baae.2014.06.006. ISSN 1439-1791.
- ↑ Kowalczyk, Rafał; Kamiński, Tomasz; Borowik, Tomasz (2021-08-15). "Do large herbivores maintain open habitats in temperate forests?". Forest Ecology and Management. 494: 119310. doi:10.1016/j.foreco.2021.119310. ISSN 0378-1127. S2CID 235513778.
- ↑ Buckland, Paul. "Can the pre-Neolithic provide suitable models for rewilding the landscape in Britain?". British Wildlife.
- ↑ "Europe – part III: Into the Holocene". The Extinctions. Retrieved 2022-04-11.
- ↑ Higham, Tom; Compton, Tim; Stringer, Chris; Jacobi, Roger; Shapiro, Beth; Trinkaus, Erik; Chandler, Barry; Gröning, Flora; Collins, Chris; Hillson, Simon; O’Higgins, Paul; FitzGerald, Charles; Fagan, Michael (November 2011). "The earliest evidence for anatomically modern humans in northwestern Europe". Nature. 479 (7374): 521–524. Bibcode:2011Natur.479..521H. doi:10.1038/nature10484. ISSN 1476-4687. PMID 22048314. S2CID 4374023.
- ↑ Nikulina, Anastasia; MacDonald, Katharine; Zapolska, Anhelina; Serge, Maria Antonia; Roche, Didier M.; Mazier, Florence; Davoli, Marco; Svenning, Jens-Christian; van Wees, Dave; Pearce, Elena A.; Fyfe, Ralph; Roebroeks, Wil; Scherjon, Fulco (2024-01-15). "Hunter-gatherer impact on European interglacial vegetation: A modelling approach". Quaternary Science Reviews. 324: 108439. doi:10.1016/j.quascirev.2023.108439. ISSN 0277-3791.
- ↑ Lemoine, Rhys Taylor; Buitenwerf, Robert; Svenning, Jens-Christian (2023-12-01). "Megafauna extinctions in the late-Quaternary are linked to human range expansion, not climate change". Anthropocene. 44: 100403. Bibcode:2023Anthr..4400403L. doi:10.1016/j.ancene.2023.100403. ISSN 2213-3054. S2CID 261650124.
- ↑ Sandom, Christopher; Faurby, Søren; Sandel, Brody; Svenning, Jens-Christian (2014-07-22). "Global late Quaternary megafauna extinctions linked to humans, not climate change". Proceedings of the Royal Society B: Biological Sciences. 281 (1787): 20133254. doi:10.1098/rspb.2013.3254. PMC 4071532. PMID 24898370.
- ↑ Bakker, Elisabeth S.; Gill, Jacquelyn L.; Johnson, Christopher N.; Vera, Frans W. M.; Sandom, Christopher J.; Asner, Gregory P.; Svenning, Jens-Christian (2016-01-26). "Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation". Proceedings of the National Academy of Sciences. 113 (4): 847–855. Bibcode:2016PNAS..113..847B. doi:10.1073/pnas.1502545112. ISSN 0027-8424. PMC 4743795. PMID 26504223.
- ↑ Manzano, Pablo; Pardo, Guillermo; Itani, Moustapha A.; del Prado, Agustín (2023-01-06). "Underrated past herbivore densities could lead to misoriented sustainability policies". npj Biodiversity. 2 (1): 1–6. doi:10.1038/s44185-022-00005-z. ISSN 2731-4243. S2CID 255670593.
- ↑ Babai, Dániel; Molnár, Zsolt (2014-01-01). "Small-scale traditional management of highly species-rich grasslands in the Carpathians". Agriculture, Ecosystems & Environment. Biodiversity of Palaearctic grasslands: processes, patterns and conservation. 182: 123–130. Bibcode:2014AgEE..182..123B. doi:10.1016/j.agee.2013.08.018. ISSN 0167-8809.
- 1 2 Feurdean, Angelica; Ruprecht, Eszter; Molnár, Zsolt; Hutchinson, Simon M.; Hickler, Thomas (2018-12-01). "Biodiversity-rich European grasslands: Ancient, forgotten ecosystems". Biological Conservation. 228: 224–232. Bibcode:2018BCons.228..224F. doi:10.1016/j.biocon.2018.09.022. ISSN 0006-3207. S2CID 91611351.
- ↑ Hardenbol, Alwin A.; Junninen, Kaisa; Kouki, Jari (2020-04-15). "A key tree species for forest biodiversity, European aspen (Populus tremula), is rapidly declining in boreal old-growth forest reserves". Forest Ecology and Management. 462: 118009. doi:10.1016/j.foreco.2020.118009. ISSN 0378-1127. S2CID 213200300.
- 1 2 3 Pärtel, M.; Bruun, H. H.; Samuul, M. (2005). "Biodiversity in temperate European grasslands: origin and conservation". Grassland Science in Europe. 10.
- ↑ Wilson, J. Bastow; Peet, Robert K.; Dengler, Jürgen; Pärtel, Meelis (August 2012). Palmer, Michael (ed.). "Plant species richness: the world records". Journal of Vegetation Science. 23 (4): 796–802. Bibcode:2012JVegS..23..796W. doi:10.1111/j.1654-1103.2012.01400.x. ISSN 1100-9233. S2CID 53548257.
- ↑ "Grassland Ecosystem – an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2022-06-25.
- ↑ Dasgupta, Shreya (June 2021). "Many Tree-Planting Campaigns Are Based on Flawed Science – The Wire Science". Retrieved 2022-06-25.
- ↑ "Atlas of Forest and Landscape Restoration Opportunities". World Resources Institute. Retrieved 2022-06-25.
- ↑ Bond, William J.; Stevens, Nicola; Midgley, Guy F.; Lehmann, Caroline E.R. (9 September 2019). "The Trouble with Trees: Afforestation Plans for Africa". Trends in Ecology & Evolution. 34 (11): 963–965. doi:10.1016/j.tree.2019.08.003. hdl:20.500.11820/ad569ac5-dc12-4420-9517-d8f310ede95e. PMID 31515117. S2CID 202568025 – via Cell Press.
- ↑ Veldman, Joseph V. (January 2015). "Tyranny of trees in grassy biomes" (PDF). Science. 347 (6221): 484–485. doi:10.1126/science.347.6221.484-c. hdl:2268/177699. PMID 25635078 – via ResearchGate.
- ↑ Warren, Martin S.; Maes, Dirk; van Swaay, Chris A. M.; Goffart, Philippe; Van Dyck, Hans; Bourn, Nigel A. D.; Wynhoff, Irma; Hoare, Dan; Ellis, Sam (2021-01-12). "The decline of butterflies in Europe: Problems, significance, and possible solutions". Proceedings of the National Academy of Sciences. 118 (2). Bibcode:2021PNAS..11802551W. doi:10.1073/pnas.2002551117. ISSN 0027-8424. PMC 7812787. PMID 33431566.
- ↑ Sydenham, Markus A. K.; Eldegard, Katrine; Venter, Zander S.; Evju, Marianne; Åström, J.; Rusch, Graciela M. (2022-04-01). "Priority maps for pollinator habitat enhancement schemes in semi-natural grasslands". Landscape and Urban Planning. 220: 104354. doi:10.1016/j.landurbplan.2022.104354. hdl:11250/3001214. ISSN 0169-2046. S2CID 245887408.
- ↑ Dvorský, Miroslav; Mudrák, Ondřej; Doležal, Jiří; Jirků, Miloslav (2022-05-01). "Reintroduction of large herbivores restored plant species richness in abandoned dry temperate grassland". Plant Ecology. 223 (5): 525–535. Bibcode:2022PlEco.223..525D. doi:10.1007/s11258-022-01225-w. ISSN 1573-5052. S2CID 246932379.
- ↑ Bonavent, Christoffer; Olsen, Kent; Ejrnæs, Rasmus; Fløjgaard, Camilla; Hansen, Morten D. D.; Normand, Signe; Svenning, Jens-Christian; Bruun, Hans Henrik (January 2023). "Grazing by semi-feral cattle and horses supports plant species richness and uniqueness in grasslands". Applied Vegetation Science. 26 (1). Bibcode:2023AppVS..26E2718B. doi:10.1111/avsc.12718. ISSN 1402-2001. S2CID 257196659.
- ↑ Köhler, Martina; Schmidt, Annika; Hölzel, Norbert; Baasch, Annett; Tischew, Sabine (2023). "Positive long-term effects of year-round horse grazing in orchid-rich dry calcareous grasslands–Results of a 12-year study". Frontiers in Ecology and Evolution. 11. doi:10.3389/fevo.2023.1107987. ISSN 2296-701X.
- ↑ Waldén, Emelie, Lindborg, Regina; Helm, Aveliina; Stockholms universitet; Naturvetenskapliga fakulteten; Landscape Ecology (2018). Restoration of semi-natural grasslands Impacts on biodiversity, ecosystem services and stakeholder perceptions. Stockholm: Department of Physical Geography, Stockholm University. ISBN 978-91-7797-172-6. OCLC 1038678595.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Vodka, Stepan; Konvicka, Martin; Cizek, Lukas (2008-12-16). "Habitat preferences of oak-feeding xylophagous beetles in a temperate woodland: implications for forest history and management". Journal of Insect Conservation. 13 (5): 553. doi:10.1007/s10841-008-9202-1. ISSN 1572-9753. S2CID 39052657.
- ↑ Wolański, Paweł; Bobiec, Andrzej; Ortyl, Bernadetta; Makuch-Pietraś, Iwona; Czarnota, Paweł; Ziobro, Jan; Korol, Mykola; Havryliuk, Serhii; Paderewski, Jakub; Kirby, Keith (2021-03-01). "The importance of livestock grazing at woodland-grassland interface in the conservation of rich oakwood plant communities in temperate Europe". Biodiversity and Conservation. 30 (3): 741–760. Bibcode:2021BiCon..30..741W. doi:10.1007/s10531-021-02115-9. ISSN 1572-9710. S2CID 234077522.
- ↑ Eriksson, Ove (2021-04-01). "The importance of traditional agricultural landscapes for preventing species extinctions". Biodiversity and Conservation. 30 (5): 1341–1357. Bibcode:2021BiCon..30.1341E. doi:10.1007/s10531-021-02145-3. ISSN 1572-9710. S2CID 233818234.
- ↑ Navarro, Laetitia M.; Pereira, Henrique M. (2012-09-01). "Rewilding Abandoned Landscapes in Europe". Ecosystems. 15 (6): 900–912. Bibcode:2012Ecosy..15..900N. doi:10.1007/s10021-012-9558-7. ISSN 1435-0629. S2CID 17140817.
- ↑ "'It is strange to see the British struggling with the beaver': why is rewilding so controversial?". the Guardian. 2017-07-03. Retrieved 2022-07-23.
- ↑ "The role of grazers and browsers". Rewilding Britain. Retrieved 2022-07-23.
- ↑ "Rewilding at Knepp". Whatifyoujustleaveit. Retrieved 2022-07-23.
- ↑ Tree, Isabella (2018). Wilding. Picador. ISBN 978-1509805105.
- ↑ "Rewilding Europe | Making Europe a Wilder Place". Rewilding Europe. Retrieved 2021-12-03.
- ↑ "Natural grazing delivering increasing benefits across Europe". Rewilding Europe. 2022-02-16. Retrieved 2022-02-16.
- ↑ Garbarino, Matteo; Bergmeier, Erwin (2014). "Plant and vegetation diversity in European wood-pastures". In Plieninger, Tobias; Hartel, Tibor (eds.). European Wood-pastures in Transition. Routledge. ISBN 978-0-8153-9531-7.
- ↑ Bergmeier, Erwin; Roellig, Marlene (2014). "Diversity, threats and conservation of European wood-pastures". In Plieninger, Tobias; Hartel, Tibor (eds.). European Wood-pastures in Transition. Routledge.
- ↑ Falk, Steven (2014). "Wood-pastures as reservoirs for invertebrates". In Plieninger, Tobias; Hartel, Tibor (eds.). European Wood-pastures in Transition. Routledge.
- ↑ Mann, Daniel H.; Groves, Pamela; Gaglioti, Benjamin V.; Shapiro, Beth A. (2019). "Climate-driven ecological stability as a globally shared cause of Late Quaternary megafaunal extinctions: the Plaids and Stripes Hypothesis". Biological Reviews. 94 (1): 328–352. doi:10.1111/brv.12456. ISSN 1469-185X. PMC 7379602. PMID 30136433.
- ↑ Zimov, S. A.; Chuprynin, V. I.; Oreshko, A. P.; Chapin, F. S.; Reynolds, J. F.; Chapin, M. C. (1995-11-01). "Steppe-Tundra Transition: A Herbivore-Driven Biome Shift at the End of the Pleistocene". The American Naturalist. 146 (5): 765–794. doi:10.1086/285824. ISSN 0003-0147. S2CID 60439469.
- ↑ "Animals | Pleistocene Park". pleistocenepark.ru. Retrieved 2022-01-06.
- ↑ "Pleistocene Park". pleistocenepark.ru. Retrieved 2022-01-06.
Further reading
- Hodder, K.H.; Bullock, J.M.; Buckland, P.C.; Kirby, K.J. (2005). Large Herbivores in the Wildwood and in modern naturalistic grazing systems (Report). English Nature.
- Kirby, K.J. (2004-05-01). "A model of a natural wooded landscape in Britain as influenced by large herbivore activity". Forestry. 77 (5): 405–420. doi:10.1093/forestry/77.5.405. ISSN 0015-752X.
- Marris, Emma (2011). Rambunctious garden : saving nature in a post-wild world (1st U.S. ed.). New York: Bloomsbury. ISBN 978-1-60819-032-4. OCLC 639161286.
- Reif, Albert; Gärtner, Stefanie (December 2007). "Die natürliche Verjüngung der laubabwerfenden Eichen-arten Stieleiche (Quercus robur L.) und Traubeneiche (Quercus petraea Liebl.) – eine Literaturstudie mit besonderer Berücksichtigung der Waldweide" (PDF). Waldoekologie Online (in German).
- Rotherham, Ian D., ed. (2013). Trees, forested landscapes, and grazing animals : a European perspective on woodlands and grazed treescapes. Abingdon, Oxon: Routledge. ISBN 978-0-415-62611-8. OCLC 829911159.
- Smith, David; Whitehouse, Nicki; Bunting, M. Jane; Chapman, Henry (March 2010). "Can we characterise 'openness' in the Holocene palaeoenvironmental record? Modern analogue studies of insect faunas and pollen spectra from Dunham Massey deer park and Epping Forest, England" (PDF). The Holocene. 20 (2): 215–229. Bibcode:2010Holoc..20..215S. doi:10.1177/0959683609350392. ISSN 0959-6836. S2CID 54514775.
- Tree, Isabella (2018). Wilding : returning nature to our farm. New York: MacMillan UK. ISBN 978-1-68137-371-3. OCLC 1080275081.
- Vera, Frans W.M. (2002-09-01). "The Dynamic European Forest". Arboricultural Journal. 26 (3): 179–211. Bibcode:2002ArbJ...26..179V. doi:10.1080/03071375.2002.9747335. ISSN 0307-1375. S2CID 129801882.

