The Wanzlick equilibrium is a chemical equilibrium between a relatively stable carbene compound and its dimer. The equilibrium was proposed to apply to certain electron-rich alkenes, such as tetraminoethylenes, which have been called "carbene dimers." Such equilibria occur, but the mechanism does not proceed simply, but requires catalysts.
Original conjecture
In 1960, Hans-Werner Wanzlick and E. Schikora proposed that carbenes derived from dihydroimidazol-2-ylidene were generated by vacuum pyrolysis of 2-trichloromethyl dihydroimidazole derivatives, with the loss of chloroform.[1][2]
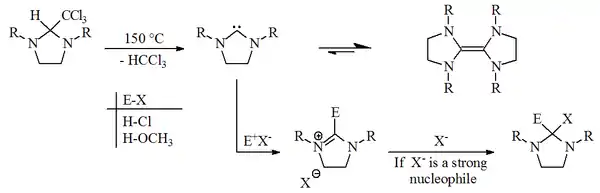
Wanzlick and Schikora believed that once prepared these carbenes existed in an unfavourable equilibrium with their corresponding dimers. This assertion was based on reactivity studies which they believed showed that the free carbene reacted with electrophiles (E-X). The dimer (a substituted tetraaminoethylene) was believed to be inactive to the electrophiles (E-X), and thought to merely act as a stable carbene reservoir.[3]
Conjecture challenged
Wanzlick's hypothesis of a carbene-dimer equilibrium was tested by David M. Lemal and others.[4][5] Heating mixtures of tetraaminoethylene derivatives did not produce mixed dimers:

This result indicates that a 'carbene-dimer equilibrium' does not occur for these dihydroimidazol-2-ylidene derivatives.
Lemal[4] proposed that Wanzlick's observations could be explained by acid-catalyzed reactions.
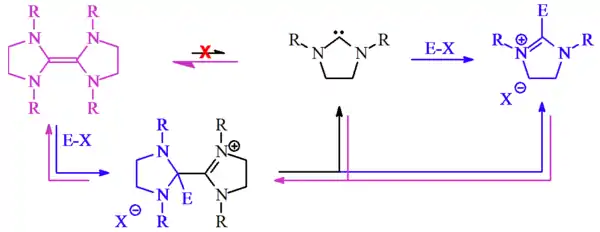
Lemal proposed that the electrophile converts the tetraaminoethylenes into cationic species. He proposed that this cation then dissociated into the free carbene plus the resultant salt. The free carbene could then either re-dimerise, regenerating the tetraaminoethylene starting material, or react with E-X (as Wanzlick originally predicted), with either route eventually giving the same reaction product, the dihydroimidazolium salt.
Conjecture confirmed
In 1999 Michael K. Denk reinvestigated the cross-over experiments that supported the Wanzlick equilibrium.[7] This report prompted Lemal to repeat his 1964 experiments. Denk's findings were confirmed only with deuterated tetrahydrofuran (THF) as a solvent. With toluene and added KH as an electrophile quencher, however, the cross-over product was again not observed.[8]
In 1999 Lemal and others[9][10] investigated an equilibrium between a dibenzotetraazafulvalene derivative and its carbene. These studies led Böhm & Herrmann to conclude in 2000 that "the Wanzlick equilibrium between a tetraaminoethylene and its corresponding carbene did exist".[11] This notion was confirmed in 2010 by Kirmse.[12]
Others subsequently showed that unhindered diaminocarbenes form dimers by acid-catalysed dimerisation as shown in the Lemal.[13]
Sublimation experiments with carbene dimers and their protonated derivatives quantify acid catalysis and corroborate that tetraaminoolefins may dissociate without adventitious protons. Acid catalysis is however required for the dissociation of triaminoolefins (NHC-CAAC dimers).[14]
References
- ↑ Wanzlick Hans-Werner; Schikora E (1960). "Ein neuer Zugang zur Carben-Chemie". Angewandte Chemie. 72 (14): 494. doi:10.1002/ange.19600721409.
- ↑ Wanzlick H. W.; Schikora E. (1960). "Ein nucleophiles Carben". Chemische Berichte. 94 (9): 2389–2393. doi:10.1002/cber.19610940905.
- ↑ H. W. Wanzlick (1962). "Aspects of Nucleophilic Carbene Chemistry". Angew. Chem. Int. Ed. Engl. 1 (2): 75–80. doi:10.1002/anie.196200751.
- 1 2 D. M. Lemal; R. A. Lovald & K. I. Kawano (1964). "Tetraaminoethylenes. The Question of Dissociation". J. Am. Chem. Soc. 86 (12): 2518–2519. doi:10.1021/ja01066a044.
- ↑ H. E. Winberg; J. E. Carnahan; D. D. Coffman & M. Brown (1965). "Tetraaminoethylenes". J. Am. Chem. Soc. 87 (9): 2055–2056. doi:10.1021/ja01087a040.
- ↑ T. A. Taton & P. Chen (1996). "A Stable Tetraazafulvalene". Angew. Chem. Int. Ed. Engl. 35 (9): 1011–1013. doi:10.1002/anie.199610111.
- ↑ Denk Michael K.; Hatanoa Ken; Maa Martin (1999). "Nucleophilic carbenes and the wanzlick equilibrium: A reinvestigation". Tetrahedron Letters. 40 (11): 2057–2060. doi:10.1016/S0040-4039(99)00164-1.
- ↑ Liu Yufa; Lemal David M (2000). "Concerning the Wanzlick equilibrium". Tetrahedron Letters. 41 (5): 599–602. doi:10.1016/S0040-4039(99)02161-9.
- ↑ Liu Yufa; Lindner Patrick E.; Lemal David M. (1999). "Thermodynamics of a Diaminocarbene−Tetraaminoethylene Equilibrium". J. Am. Chem. Soc. 121 (45): 10626–10627. doi:10.1021/ja9922678.
- ↑ Hahn F. Ekkehardt; Wittenbecher Lars; Le Van Duc; Fröhlich Roland (2000). "Evidence for an Equilibrium between an N-heterocyclic Carbene and Its Dimer in Solution". Angewandte Chemie International Edition. 39 (3): 541–544. doi:10.1002/(sici)1521-3773(20000204)39:3<541::aid-anie541>3.0.co;2-b. Archived from the original on 2013-01-05.
- ↑ Böhm Volker P. W.; Herrmann Wolfgang A. (2000). "The Wanzlick Equilibrium". Angewandte Chemie. 39 (22): 4036–4038. doi:10.1002/1521-3773(20001117)39:22<4036::AID-ANIE4036>3.0.CO;2-L.
- ↑ Kirmse W (2010). "The Beginnings of N-Heterocyclic Carbenes". Angewandte Chemie International Edition. 49 (47): 8798–8801. doi:10.1002/anie.201001658.
- ↑ Roger W. Alder; Leila Chaker; François P. V. Paolini (2004). "Bis(diethylamino)carbene and the mechanism of dimerisation for simple diaminocarbenes". Chem. Commun. (19): 2172–2173. doi:10.1039/b409112d. PMID 15467857.
- ↑ J. Messelberger; M. Kumar; S. J. Goodner; D. Munz (2021). "Wanzlick's equilibrium in tri- and tetraaminoolefins". Org. Chem. Front. 8: 6663–6669. doi:10.1039/D1QO01320C.