 | |
| Names | |
|---|---|
| IUPAC name
Silylgermane | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
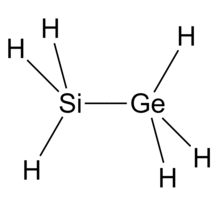
| SiH3GeH3 | |
| Molar mass | 106.763 g·mol−1 |
| Appearance | Colorless gas |
| Odor | Unpleasant, irritating[3] |
| Melting point | −119.7 °C (−183.5 °F; 153.5 K)[4] |
| Boiling point | 7.0 °C (44.6 °F; 280.1 K)[4] |
| Structure | |
| Ethane-like | |
| Hazards[2] | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Extremely flammable, toxic and corrosive, may cause severe and permanent eye damage, fatal if inhaled |
Inhalation hazards |
Fatal |
Eye hazards |
Permanent eye damage |
Skin hazards |
Corrosive injuries |
| GHS labelling: | |
    | |
| Danger | |
| H220, H280, H314, H330 | |
| P210, P222, P230, P260, P264, P271, P280, P284, P301+P330+P331, P302, P304+P340, P305, P316, P320, P321, P338, P361, P363, P377, P381, P403, P403+P233, P405, P410+P403, P501 | |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Silylgermane is an inorganic compound with the chemical formula H3Si−GeH3. It is a colorless gas with an unpleasant odor. It is unstable in air. It is very flammable, very toxic and corrosive. It reacts with alkali liberating hydrogen.[2][5][4][3]
History
Silylgermane was prepared in 1962 by Alan MacDiarmid and his coworkers by a silent electric discharge through an equimolar mix of silane and germane gases for a total of 28 hours. This method produces other hydrides of silicon and germanium as well.[4][3]
Preparation
One of the most common methods of preparation of silylgermane is the reaction of germane (GeH4) and chlorosilane (SiH3Cl) in the presence of a catalyst. Another method is the reaction of germane and silicon tetrachloride in the presence of a reducing agent. Silylgermane can also be produced by the reaction between hydrochloric acid and magnesium or calcium germanides-silicides, and by the reaction between hydrofluoric acid and a mixture of silicon monoxide and germanium monoxide. The synthesis of silylgermane requires careful handling due to its extreme toxicity and flammability.[3]
Structure
Silylgermane molecule contains both silicon and germanium atoms.[6] The molecule of silylgermane can be viewed as a silyl group replacing one of the hydrogen atoms of the germane molecule. According to the VSEPR theory, the molecule structure is like ethane, with a tetrahedral molecular geometry on silicon and germanium atoms. Silylgermane is a group 14 hydride.[7][8][4]
Uses
Silylgermane is useful in scientific research applications, for example in research of the properties of silicon and germanium compounds. Silylgermane is used as a precursor for the synthesis of germanium compounds, as well as in electronics such as germanium quantum dots, germanium nanowires, and in the production of solar cells and semiconductors.[9]
Safety
Silylgermane is extremely flammable and may explode, especially upon heating closed bottles containing this chemical. Silylgermane is known to be very toxic, thus, it is important to handle this chemical with cautiousness and to use appropriate protective equipment. It irritates skin, eyes and respiratory system. It is corrosive both to materials and living tissues (e.g. skin, mucous membrane, lungs and eyes). May cause severe and permanent skin and eye damage. Inhalation of silylgermane and its fumes may cause death.[2]
Derivatives
Derivatives of the general formula X3Si−GeX3 (X = hydrogen, halogen, alkyl, aryl, and mixtures of these groups) are called silylgermanes as well.
Organic silylgermanes R3Si−GeR'3 can be prepared by the reaction between silylpotassium R3SiK and chlorogermane R'3GeCl, or by the reaction between germylpotassium R'3GeK and chlorosilane R3SiCl.[3]
- R3SiK + R'3GeCl → R3SiGeR'3 + KCl
- R'3GeK + R3SiCl → R3SiGeR'3 + KCl
The way to obtain fully alkylated silylgermanes is reaction between trialkylsilyl halide R3SiX, trialkylgermyl halide R'3GeX and the sodium metal (typically, the R and R' groups are ethyl and the X is bromine Br).[3]
- R3SiX + R'3GeX + 2 Na → R3SiGeR'3 + 2 NaX
References
- ↑ "Germyl - silyl (1:1) | H6GeSi | ChemSpider".
- 1 2 3 4 "Silylgermane".
- 1 2 3 4 5 6 Organosilicon Heteropolymers and Heterocompounds. Springer. 6 December 2012. ISBN 9781461586272.
- 1 2 3 4 5 Spanier, Edward J.; MacDiarmid, Alan G. (1963). "The Synthesis of Germylsilane from Silane and Germane in a Silent Electric Discharge". Inorganic Chemistry. 2: 215–216. doi:10.1021/ic50005a055.
- ↑ "13768-63-3, Silylgermane, CAS No 13768-63-3 Silylgermane".
- ↑ Roy, Matthew M. D.; Omaña, Alvaro A.; Wilson, Andrew S. S.; Hill, Michael S.; Aldridge, Simon; Rivard, Eric (2021). "Molecular Main Group Metal Hydrides". Chemical Reviews. 121 (20): 12784–12965. doi:10.1021/acs.chemrev.1c00278. PMID 34450005. S2CID 237341726.
- ↑ Petrucci, R. H.; W. S., Harwood; F. G., Herring (2002). General Chemistry: Principles and Modern Applications (8th ed.). Prentice-Hall. pp. 413–414 (Table 11.1). ISBN 978-0-13-014329-7.
- ↑ https://goldbook.iupac.org/
- ↑ Ritter, Cole J.; Hu, Changwu; Chizmeshya, Andrew V. G.; Tolle, John; Klewer, Douglas; Tsong, Ignatius S. T.; Kouvetakis, John (2005). "Synthesis and Fundamental Studies of (H3Ge) x SiH4- x Molecules: Precursors to Semiconductor Hetero- and Nanostructures on Si". Journal of the American Chemical Society. 127 (27): 9855–9864. doi:10.1021/ja051411o. PMID 15998091.