| Pseudocercosporella capsellae | |
|---|---|
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Subclass: | |
| Order: | |
| Family: | |
| Genus: | Pseudocercosporella |
| Species: | P. capsellae |
| Binomial name | |
| Pseudocercosporella capsellae (Ellis & Everh.) Deighton,(1973) | |
| Synonyms | |
|
Cercoseptoria capsellae | |
_(14578947747).jpg.webp)
Pseudocercosporella capsellae is a plant pathogen infecting crucifers (canola, mustard, rapeseed). P. capsellae is the causal pathogen of white leaf spot disease, which is an economically significant disease in global agriculture. P. capsellae has a significant affect on crop yields on agricultural products, such as canola seed and rapeseed.[1] Researchers are working hard to find effective methods of controlling this plant pathogen, using cultural control, genetic resistance, and chemical control practices. Due to its rapidly changing genome,[2] P. capsellae is a rapidly emerging plant pathogen that is beginning to spread globally and affect farmers around the world.[3]
Habitat and Geographical Distribution
Habitat
Pseudocercosporella capsellae is generally found in humid environments. When P. capsellae is found in environments with low humidity, the fungus is unable to germinate and cause disease.[3] This pathogen is not a thermophile, explaining how it is found in temperate climates without extreme heat. After introduction into an area, P. capsellae is found in most neighboring Brassicaceae agricultural fields. In the wild, P. capsellae can be observed in prairie environments containing mustard weed.[4]
Geographical Distribution
P. capsellae has been identified on four of the seven continents of the world: North America, Europe, Asia, and Australia. Specifically, P. capsellae has been found in agricultural fields in China, Japan, Canada, India, Australia, the Pacific Northwest region of the United States, the United Kingdom, France, Poland, and Scandinavian nations.[3]
Morphology and Microscopic Features
P. capsellae is an ascomycete, meaning it produces ascospores housed in asci as means of sexual reproduction. Sexual structures are found in the sexual stage of this fungus, which has been classified as Mycosphaerella capsellae. The ascocarp of M. capsellae is a cleistothecium, meaning asci are shielded from the environment prior to ascospore release. As means of asexual reproduction, P. capsellae produces chains of septate conidia. Conidia range in size between about 42-71μm in length and about 3μm in width.[5] These chains of conidia are attached to a long conidiophore and stipe, connecting these asexual structures to the sterile hyphal network of the fungal body. In culture, P. capsellae appears black and white on potato dextrose agar (PDA). When observed under a microscope, P. capsellae appears a reddish-purple color due to the fungus' production of a purple-pink pigment.[6] P. capsellae also is known to produce a mycotoxin, cercosporin, which increases the virulence of the pathogen.[6]
Disease Signs/Symptoms, Cycle, and Control
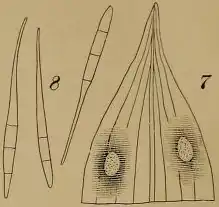
Disease Signs and Symptoms
Infected crucifers display white lesions on leaves when infected by P. capsellae. These white lesions oftentimes have nonuniform shapes, and darken as the fungus matures on its host.[7] Lesions on leaves initially can be 1-2mm in diameter, but can grow up to 10mm in diameter as the disease progresses.[8] Leaves can fall off of host plants if infection is severe and widespread throughout a particular host.[7] Gray or tan lesions may also appear on host stems; these lesions oftentimes harbor the sexual stage of P. capsellae, where ascospores are developed and released.[9] Conidia can be found on the underside of leaves, oftentimes in locations corresponding to where lesions are present.[3]
Disease Cycle
Conidia from the asexual structures of P. capsellae germinate at optimal temperatures of 20-24°C.[3] At these temperate conditions and in ample humidity, conidia can be spread to new host plants via wind, water droplet splash, or by improperly sanitized farm equipment.[10] These conidia penetrate new host leaves or stems and create infection. Crucifers, such as canola or rapeseed, are the primary host for this pathogen. In rare cases, cover crops or neighboring species of weeds can act as secondary hosts for the sexual stage of P. capsellae.[3] P. capsellae overwinters as thick-walled mycelium on infected detritus in fields, and germinates again to infect new hosts as conditions become more ideal for spread.[11] P. capsellae is a hemibiotroph, as indicated by its ability to keep host crucifers alive until host leaves fall off during severe infection.
Control Strategies
Many management strategies have been implemented in attempt to control the spread of P. capsellae. One common method of control is the use of fungicides as means of chemical control. The use of fungicides has been discovered to be ineffective at the control of P. capsellae, as this pathogen is resistant to most of the common fungicides utilized by farmers.[12]
Cultural control methods are the most common management strategy that farmers use against P. capsellae. Methods such as crop rotation, proper sanitation of farm equipment, and planting crucifer crops with more space in between crops are effective methods of managing the spread of P. capsellae in fields.[3] Sanitation of farm equipment and crop rotation are methods of reducing initial inoculum of conidia produced by P. capsellae.
Breeding genetic resistance towards P. capsellae is a promising method for disease management of this pathogen. Researchers across the world have been conducting genetic crosses of Brassica crops to find resistance genes that can make crops less susceptible to P. capsellae infection.[2] Although this method of control is promising, P. capsellae has a genome that is rapidly changing, making it difficult for researchers to identify host resistance genes that remain effective against P. capsellae for substantial periods of time.[2]

References
- ↑ Murtza, Tamsal; You, Ming Pei; Barbetti, Martin J. (2021-05-01). "Canola Growth Stage at Time of Infection Determines Magnitude of White Leaf Spot (Neopseudocercosporella capsellae) Impact". Plant Disease. 105 (5): 1515–1521. doi:10.1094/PDIS-09-20-2036-RE. ISSN 0191-2917. PMID 33185518. S2CID 226854625.
- 1 2 3 Inturrisi, Fabian C.; Barbetti, Martin J.; Tirnaz, Soodeh; Patel, Dhwani A.; Edwards, David; Batley, Jacqueline (2020-09-30). "Molecular characterization of disease resistance in Brassica juncea – The current status and the way forward". Plant Pathology. 70 (1): 13–34. doi:10.1111/ppa.13277. ISSN 0032-0862. S2CID 224912221.
- 1 2 3 4 5 6 7 Gunasinghe, Niroshini; Barbetti, Martin J.; You, Ming Pei; Burrell, Daniel; Neate, Stephen (2020). "White Leaf Spot Caused by Neopseudocercosporella capsellae: A Re-emerging Disease of Brassicaceae". Frontiers in Cellular and Infection Microbiology. 10: 588090. doi:10.3389/fcimb.2020.588090. ISSN 2235-2988. PMC 7655544. PMID 33194833.
- ↑ Petrie, G.A.; Vanterpool, T.C. (1978). "Pseudocercosporella capsellae, the cause of white leaf spot and grey stem of Cruciferae in Western Canada" (PDF). Canadian Plant Disease Survey. 58: 69–72.
- ↑ Eshraghi, L.; You, M. P.; Barbetti, M. J. (October 2005). "First Report of White Leaf Spot Caused by Pseudocercosporella capsellae on Brassica juncea in Australia". Plant Disease. 89 (10): 1131. doi:10.1094/PD-89-1131B. ISSN 0191-2917. PMID 30791294.
- 1 2 Gunasinghe, Niroshini; You, Ming Pei; Cawthray, Gregory R.; Barbetti, Martin J. (August 2016). "Cercosporin From Pseudocercosporella capsellae and its Critical Role in White Leaf Spot Development". Plant Disease. 100 (8): 1521–1531. doi:10.1094/PDIS-10-15-1192-RE. ISSN 0191-2917. PMID 30686233.
- 1 2 Department of Jobs, Precincts and Regions (2020-07-16). "Canola diseases - Agriculture". Agriculture Victoria. Retrieved 2022-05-08.
- ↑ "White Leaf Spot and Grey Stem | Canola Encyclopedia". The Canola Council of Canada. Retrieved 2022-05-08.
- ↑ Inman, A.J.; Fitt, B.D.L (2007). Compendium of Brassica Diseases. St. Paul, MN: APS Press. pp. 50–54.
- ↑ "Cercospora Leaf Spot". CropWatch. 2015-09-18. Retrieved 2022-05-08.
- ↑ "Cercospora Leaf Spot of Table beet". www.vegetables.cornell.edu. Retrieved 2022-05-08.
- ↑ Ware, Sarah B. (2006). Aspects of sexual reproduction in Mycosphaerella species on wheat and barley : genetic studies on specificity, mapping, and fungicide resistance (Thesis). [publisher not identified]. ISBN 90-8504-527-4. OCLC 76891618.