 | |
| Names | |
|---|---|
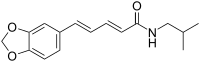
| Preferred IUPAC name
(2E,4E)-5-(2H-1,3-Benzodioxol-5-yl)-N-(2-methylpropyl)penta-2,4-dienamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C16H19NO3 | |
| Molar mass | 273.332 g·mol−1 |
| Melting point | 167-169 °C (332.6-336.2 °F; 440-442K) |
| Solubility | DMSO |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Piperlonguminine is a alkaloid amide isolated from Piper longum.[1][2]
References
- ↑ Kim, KS; Kim, JA; Eom, SY; Lee, SH; Min, KR; Kim, Y (February 2006). "Inhibitory effect of piperlonguminine on melanin production in melanoma B16 cell line by downregulation of tyrosinase expression". Pigment Cell Research. 19 (1): 90–8. doi:10.1111/j.1600-0749.2005.00281.x. PMID 16420250.
- ↑ Lee, W; Yoo, H; Kim, J. A; Lee, S; Jee, J. G; Lee, M. Y; Lee, Y. M; Bae, J. S (2013). "Barrier protective effects of piperlonguminine in LPS-induced inflammation in vitro and in vivo". Food and Chemical Toxicology. 58: 149–57. doi:10.1016/j.fct.2013.04.027. PMID 23619565.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.