 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C31H29O14+ | |
| Molar mass | 625.55 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
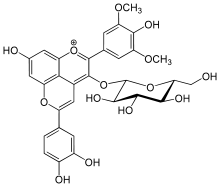
Pinotin A is a pinotin, a type of pyranoanthocyanins and a class of phenolic compounds found in red wine.[1][2]
See also
- Pinotin A aglycone[3]
- Phenolic content in wine
References
- ↑ Investigations on Anthocyanins in Wines from Vitis vinifera cv. Pinotage: Factors Influencing the Formation of Pinotin A and Its Correlation with Wine Age. Schwarz Michael, Hofmann Glenn and Winterhalter Peter, J. Agric. Food Chem., 2004, 52 (3): 498–504. doi:10.1021/jf035034f
- ↑ Variation of pyranoanthocyanins in red wines of different varieties and vintages and the impact of pinotin A addition on their color parameters. Michael Rentzsch, Fabian Weber, Dominik Durner, Ulrich Fischer and Peter Winterhalter, European Food Research and Technology, Volume 229, Number 4, pp. 689-696, doi:10.1007/s00217-009-1102-4
- ↑ "Showing dietary polyphenol Pinotin a aglycone - Phenol-Explorer".
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.