N-acylethanolamine acid amide hydrolase (NAAA) EC 3.5.1.-[1] is a member of the choloylglycine hydrolase family, a subset of the N-terminal nucleophile hydrolase superfamily. NAAA has a molecular weight of 31 kDa. The activation and inhibition of its catalytic site is of medical interest as a potential treatment for obesity and chronic pain. While it was discovered within the last decade, its structural similarity to the more familiar acid ceramidase (AC) and functional similarity to fatty acid amide hydrolase (FAAH) allow it to be studied extensively.
Mechanism
The overall enzyme mechanism involves cleavage into two chains, one of which contains the catalytic nucleophile, believed to be a cysteine residue. Unlike FAAH, which operates in basic conditions, this enzyme must operate under acidic conditions (pH ~4.5), and is completely inactivated at a pH of 8. Selective inhibitors of NAAA are ester and amide compounds, such as N-cyclohexanecarbonylpentadecylamine. NAAA is cleaved proteolytically at residue Cys-126. NAAA cleaves C-N non-peptide bonds in linear amides, particularly ethanolamides. Its mechanism is quite similar to that of AC, which is further supported by AC's ability to cleave N-acylethanolamines (NAEs), albeit at far lower rates and with different specificities. While mechanistic details are not very well known, catalytic activity of NAAA is thought to be activated by Cys-126 and Asp-145.
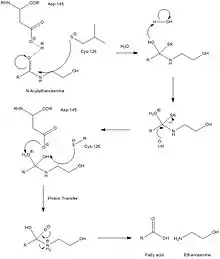
Structure
Currently, there is little information about NAAA's tertiary structure, due to its low homology to any of the other enzymes in the choloylglycine hydrolase family for which there is significant 3-dimensional structural information. In humans, this enzyme contains 359 residues. NAAA's primary structure is nearly identical to that of acid ceramidase (AC), whose only difference is the substitution of Leu at the 334th residue, for phenylalanine. The enzyme must be N-glycosylated at six sites in order for the enzyme to operate at its maximum activity level, all of which have the peptide sequence, Asn-Xxx-Ser/Thr. Unlike AC, NAAA does not form a heterodimer through disulfide bonds, but rather remains active as two separate cleaved peptides following hydrolysis.
Biological function
Fatty acid ethanolamines (FAEs) perform several physiological functions, most notably serving as messengers for pain and inflammation. NAAA's are found primarily in the lysosomal compartment of macrophages, in line with most inflammation-related proteins. The gene that codes for the protein is 4q21.1. There, they perform FAE hydrolysis, the final step in the signaling cascade for pain and inflammation, yielding an ethanolamine and a fatty acid. While it processes the cleavage of many different substrates, NAAA is most active with the substrate N-palmitoylethanolamine, suggesting that this is one of the key messengers of pain. NAAA activity in rats is highest in the lungs, while in humans it is highest in the liver, so there is cross-species variability in the enzyme's selective activity.
Disease relevance
Recent studies suggest that NAAA has significance in two widespread human conditions: chronic pain and obesity. Current research focuses on inhibiting the NAAA hydrolytic active site in order to control inflammation. It is still ambiguous as to whether reduced inflammation is correlated to reduced pain. ARN077, a β-lactone, has been one of the most intensely tested NAAA inhibitors, with the strongest promise of inhibition, as it blocks the catalytic cysteine via a thioester bond. The lack of homology between NAAA and FAAH makes NAAA-specific targeting drugs far more feasible. However, because the fatty acid concentration circulating throughout one's bloodstream is positively correlated with obesity, decreased NAAA activity is thought to be correlated with obesity.
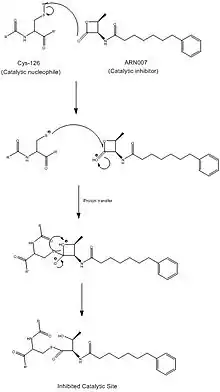
Industrial relevance
While no drugs targeting NAAA have entered the market yet, there is currently substantial research being done on the activation, and specific targeting and inhibition of NAAA. Activation of NAAA is spurred by the addition of phospholipids, and targeted inhibition by different buffers. Findings in these areas may be able to develop drugs to combat chronic pain and obesity.
Evolution
While NAAA operates much like fatty acid amide hydrolase (HUGO gene symbol: FAAH), the two enzymes are not homologous.
On the other hand, NAAA is homologous to acid ceramidase (HUGO gene symbol: ASAH1), sharing 30% sequence identity at the amino acid level in humans ENSEMBL.
Historical significance
NAAA was discovered in 2007 as an alternative source of anandamide hydrolysis. Previously, FAAH was the only known enzyme to be responsible for the degradation of these endocannabinoids, functioning in a pH range of 8.5-10. NAAA's discovery served as an explanation for endocannabinoids and anti-inflammatory ethanolamines in acidic environments, as its peak functionality is found at a pH ~4.5-5. Because of its functional role similar to FAAH, it offers another option for drug development.
See also
References
- Kazuhito Tsuboi, Naoko Takezaki & Natsuo Ueda (August 2007). "The N-acylethanolamine-hydrolyzing acid amidase (NAAA)". Chemistry & Biodiversity. 4 (8): 1914–1925. doi:10.1002/cbdv.200790159. PMID 17712833. S2CID 32163665.
- Yong-Xin Sun, Kazuhito Tsuboi, Li-Ying Zhao, Yasuo Okamoto, Didier M. Lambert & Natsuo Ueda (October 2005). "Involvement of N-acylethanolamine-hydrolyzing acid amidase in the degradation of anandamide and other N-acylethanolamines in macrophages". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1736 (3): 211–220. CiteSeerX 10.1.1.321.2849. doi:10.1016/j.bbalip.2005.08.010. PMID 16154384.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kazuhito Tsuboi, Yong-Xin Sun, Yasuo Okamoto, Nobukazu Araki, Takeharu Tonai & Natsuo Ueda (March 2005). "Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase". The Journal of Biological Chemistry. 280 (12): 11082–11092. doi:10.1074/jbc.M413473200. PMID 15655246.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Martin Kaczocha, Sherrye T. Glaser, Janiper Chae, Deborah A. Brown & Dale G. Deutsch (January 2010). "Lipid droplets are novel sites of N-acylethanolamine inactivation by fatty acid amide hydrolase-2". The Journal of Biological Chemistry. 285 (4): 2796–2806. doi:10.1074/jbc.M109.058461. PMC 2807334. PMID 19926788.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Valerio Chiurchiu, Luca Battistini & Mauro Maccarrone (January 2015). "Endocannabinoid signaling in innate and adaptive immunity". Immunology. 144 (3): 352–364. doi:10.1111/imm.12441. PMC 4557672. PMID 25585882.
- Yi Zhang, Gabriele E. Sonnenberg, Tesfaye Mersha Baye, Jack Littrell, Jennifer Gunnell, Ann DeLaForest, Erin MacKinney, Cecilia J. Hillard, Ahmed H. Kissebah, Michael Olivier & Russell A. Wilke (December 2009). "Obesity-related dyslipidemia associated with FAAH, independent of insulin response, in multigenerational families of Northern European descent". Pharmacogenomics. 10 (12): 1929–1939. doi:10.2217/pgs.09.122. PMC 3003434. PMID 19958092.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Didier M. Lambert & Giulio G. Muccioli (November 2007). "Endocannabinoids and related N-acylethanolamines in the control of appetite and energy metabolism: emergence of new molecular players". Current Opinion in Clinical Nutrition and Metabolic Care. 10 (6): 735–744. doi:10.1097/MCO.0b013e3282f00061. PMID 18089956. S2CID 17639044.
- Oscar Sasso, Guillermo Moreno-Sanz, Cataldo Martucci, Natalia Realini, Mauro Dionisi, Luisa Mengatto, Andrea Duranti, Glauco Tarozzo, Giorgio Tarzia, Marco Mor, Rosalia Bertorelli, Angelo Reggiani & Daniele Piomelli (March 2013). "Antinociceptive effects of the N-acylethanolamine acid amidase inhibitor ARN077 in rodent pain models". Pain. 154 (3): 350–360. doi:10.1016/j.pain.2012.10.018. PMC 3723234. PMID 23218523.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Jay M. West, Nikolai Zvonok, Kyle M. Whitten, Jodianne T. Wood & Alexandros Makriyannis (February 2012). "Mass spectrometric characterization of human N-acylethanolamine-hydrolyzing acid amidase". Journal of Proteome Research. 11 (2): 972–981. doi:10.1021/pr200735a. PMC 3706083. PMID 22040171.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Janani Ravi, Amita Sneh, Konstantin Shilo, Mohd W. Nasser & Ramesh K. Ganju (May 2014). "FAAH inhibition enhances anandamide mediated anti-tumorigenic effects in non-small cell lung cancer by downregulating the EGF/EGFR pathway". Oncotarget. 5 (9): 2475–2486. doi:10.18632/oncotarget.1723. PMC 4058020. PMID 24811863.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Tatsuya Tai, Kazuhito Tsuboi, Toru Uyama, Kim Masuda, Benjamin F. Cravatt, Hitoshi Houchi & Natsuo Ueda (May 2012). "Endogenous molecules stimulating N-acylethanolamine-hydrolyzing acid amidase (NAAA)". ACS Chemical Neuroscience. 3 (5): 379–385. doi:10.1021/cn300007s. PMC 3382453. PMID 22860206.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Andrea Armirotti, Elisa Romeo, Stefano Ponzano, Luisa Mengatto, Mauro Dionisi, Claudia Karacsonyi, Fabio Bertozzi, Gianpiero Garau, Glauco Tarozzo, Angelo Reggiani, Tiziano Bandiera, Giorgio Tarzia, Marco Mor & Daniele Piomelli (May 2012). "beta-Lactones Inhibit N-acylethanolamine Acid Amidase by S-Acylation of the Catalytic N-Terminal Cysteine". ACS Medicinal Chemistry Letters. 3 (5): 422–426. doi:10.1021/ml300056y. PMC 4025845. PMID 24900487.
{{cite journal}}: CS1 maint: multiple names: authors list (link)