 | |
| Names | |
|---|---|
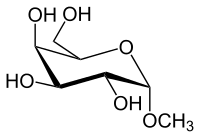
| IUPAC name
Methyl α-D-galactopyranoside | |
| Systematic IUPAC name
(2R,3R,4S,5R,6S)-2-(Hydroxymethyl)-6-methoxyoxane-3,4,5-triol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.020.229 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H14O6 | |
| Molar mass | 194.183 g·mol−1 |
| Melting point | 114-115 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Methyl-α-D-galactose is a constituent of Eleutherococcus senticosus.[1][2][3]
References
- ↑ No authors Listed (2021-05-17). Eleuthero. NBK501806 (1st ed.). Drugs and Lactation Database. p. 1. PMID 30000865.
- ↑ Bai Y, Tohda C, Zhu S, Hattori M, Komatsu K (July 2011). "Active components from Siberian ginseng (Eleutherococcus senticosus) for protection of amyloid β(25-35)-induced neuritic atrophy in cultured rat cortical neurons". Journal of Natural Medicines. 65 (3–4): 417–423. doi:10.1007/s11418-011-0509-y. PMID 21301979. S2CID 9716082.
- ↑ "Methyl-alpha-d-galactose". pubchem.ncbi.nlm.nih.gov. National Library of Medicine. Archived from the original on 2022-08-20. Retrieved 2022-08-20.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.