 | |
| Clinical data | |
|---|---|
| Trade names | Mafropan |
| AHFS/Drugs.com | Monograph |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
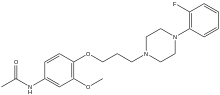
| Formula | C22H28FN3O3 |
| Molar mass | 401.482 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mafoprazine is an antipsychotic of the phenylpiperazine class which is used in veterinary medicine.[1] Intramuscular injections of mafoprazine mesylate are used for the sedation of pigs either on its own,[2] or in combination with sodium pentobarbital[3] or thiopental.[4]
Pharmacology
| Site | Ki (nM) | Species | Ref |
|---|---|---|---|
| D2 | 10.7 | Rat | [5][6] |
| α1 | 12.7 | Rat | [5][6] |
| α2 | 101.0 | Rat | [5][6] |
It demonstrates activity as a D2 dopamine receptor antagonist, an α1 adrenergic receptor antagonist, and an α2 adrenergic receptor agonist.[5]
The affinity of mafoprazine for D2 dopamine receptors is 6 and 16 times lower than that of chlorpromazine and haloperidol, respectively, but 2 times higher than that of azaperone.[5]
The Ki for various receptors was determined using rat neuronal receptor binding assays.
History
Mafoprazine was first synthesized in 1988.[5] It is sold as Mafropan® by DS Pharma Animal Health Co. Ltd., Osaka, Japan.
References
- ↑ "Mafoprazine | Chemical Substance Information | J-GLOBAL". jglobal.jst.go.jp.
- ↑ Heishima, Kazuki; Kuo, Kendon; Kimura, Masashi; Mori, Takashi (2019). Animal Lymphocyte Metaphase Chromosome Preparation. Methods in Molecular Biology. Vol. 1984. pp. 7–22. doi:10.1007/978-1-4939-9432-8_2. ISBN 978-1-4939-9430-4. S2CID 195787061.
{{cite book}}:|journal=ignored (help) - ↑ Azizi, AFN; Miyazaki, R; Yumito, T; Ohashi, Y; Uno, S; Miyajima, U; Kumamoto, M; Uchiyama, S; Yasuda, M (1 January 2018). "Effect of maternal supplementation with seaweed powder on immune status of liver and lymphoid organs of piglets". The Journal of Veterinary Medical Science. 80 (1): 8–12. doi:10.1292/jvms.17-0537. PMC 5797852. PMID 29142150.
- ↑ Umeyama, Kazuhiro; Watanabe, Kota; Watanabe, Masahito; Horiuchi, Keisuke; Nakano, Kazuaki; Kitashiro, Masateru; Matsunari, Hitomi; Kimura, Tokuhiro; Arima, Yoshimi; Sampetrean, Oltea; Nagaya, Masaki; Saito, Masahiro; Saya, Hideyuki; Kosaki, Kenjiro; Nagashima, Hiroshi; Matsumoto, Morio (14 April 2016). "Generation of heterozygous fibrillin-1 mutant cloned pigs from genome-edited foetal fibroblasts". Scientific Reports. 6 (1): 24413. Bibcode:2016NatSR...624413U. doi:10.1038/srep24413. PMC 4830947. PMID 27074716. S2CID 22352477.
- 1 2 3 4 5 6 Fukuchi, Isao; Kawashima, Kazutaka; Matsuoka, Yuzo; Ishida, Ryuichi (1988). "Neurochemical study of mafoprazine, a new phenylpiperazine derivative". The Japanese Journal of Pharmacology. 47 (1): 51–61. doi:10.1254/jjp.47.51. PMID 3411821. S2CID 13158367.
- 1 2 3 "NCATS Inxight Drugs — MAFOPRAZINE". drugs.ncats.io.