 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H24N2O3 |
| Molar mass | 352.434 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
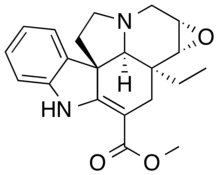
Lochnericine is a major monoterpene indole alkaloid present in the roots of Catharanthus roseus. It is also present in Tabernaemontana divaricata.[1]
Chemistry
Synthesis
Lochnericine is formed from stereoselective epoxidation of carbons 6 and 7 of tabersonine.[1]
Derivatives
See also
- Pericine
- Pervine
- Tabersonine
- Vincamine
References
- 1 2 Carqueijeiro I, Brown S, Chung K, Dang TT, Walia M, Besseau S, et al. (August 2018). "Two Tabersonine 6,7-Epoxidases Initiate Lochnericine-Derived Alkaloid Biosynthesis in Catharanthus roseus". Plant Physiology. 177 (4): 1473–1486. doi:10.1104/pp.18.00549. PMC 6084683. PMID 29934299.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.