 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
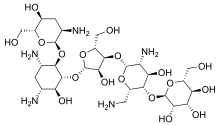
| Formula | C29H55N5O19 |
| Molar mass | 777.775 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lividomycin is a broad-spectrum aminoglycoside antibiotic.[1] It is effective against most gram positive and gram negative bacteria including Mycobacterial tuberculosis (the causative agent of tuberculosis) and Pseudomonas aeruginosa.[2]
References
- ↑ Kobayashi F, Yamaguchi M, Mitsuhashi S (January 1972). "Activity of lividomycin against Pseudomonas aeruginosa: its inactivation by phosphorylation induced by resistant strains". Antimicrobial Agents and Chemotherapy. 1 (1): 17–21. doi:10.1128/AAC.1.1.17. PMC 444159. PMID 4207755.
- ↑ Kobayashi F, Nagoya T, Yoshimura Y, Kaneko K, Ogata SI (February 1972). "Studies on New Antibiotic Lividomycins. V In vitro and in vivo antimicrobial activity of lividomycin A". The Journal of Antibiotics. 25 (2): 128–136. doi:10.7164/antibiotics.25.128. PMID 4624613.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.