 | |
| Clinical data | |
|---|---|
| Trade names | Zefnart |
| Other names | M-732; piritetrate |
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
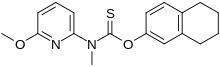
| Formula | C18H20N2O2S |
| Molar mass | 328.43 g·mol−1 |
Liranaftate (trade name Zefnart) is a topical antifungal drug.[1] It is used as a 2% cream used to treat tinea pedis (athlete's foot), tinea corporis (ringworm), and tinea cruris (jock itch).[2] It was approved for use in Japan in August 2000.[3][4]
Liranaftate works by inhibiting the fungal enzyme squalene epoxidase that is necessary for the fungus to synthesize sterols which are essential for cell membrane integrity.[5]
References
- ↑ Koga H, Nanjoh Y, Makimura K, Tsuboi R (2009). "In vitro antifungal activities of luliconazole, a new topical imidazole". Medical Mycology. 47 (6): 640–7. doi:10.1080/13693780802541518. PMID 19115136.
- ↑ "Torii Pharmaceutical to Launch Antifungal Agent for External Use, "ZEFNART SOLUTION 2%", in Japan" (Press release). Torii Pharmaceutical Co. Retrieved June 27, 2021.
- ↑ "Liranaftate". ncats.io. Retrieved June 27, 2021.
- ↑ "Liranaftate". Adis Insight. Retrieved June 27, 2021.
- ↑ "Liranaftate". targetmol.com. Retrieved June 27, 2021.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.