 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
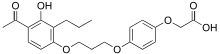
| Formula | C22H26O7 |
| Molar mass | 402.443 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
L-165041 is a phenyloxyacetate PPARδ receptor agonist. It is less potent and PPARδ selective than GW 501516.[2]
See also
References
- ↑ Iwashita A, Muramatsu Y, Yamazaki T, Muramoto M, Kita Y, Yamazaki S, et al. (March 2007). "Neuroprotective efficacy of the peroxisome proliferator-activated receptor delta-selective agonists in vitro and in vivo". The Journal of Pharmacology and Experimental Therapeutics. 320 (3): 1087–96. doi:10.1124/jpet.106.115758. PMID 17167170. S2CID 9595370.
- ↑ Atta-ur-Rahman, Reitz AB (2005). Frontiers in Medicinal Chemistry, Volume 2. Bentham Science Publishers. pp. 244–. ISBN 978-1-608-05205-9. Retrieved 2 April 2013.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.