 | |
| Clinical data | |
|---|---|
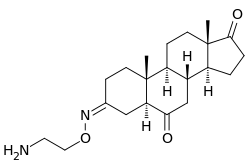
| Other names | (3Z,5α)-3-[(2-Aminoethoxy)imino]androstane-6,17-dione |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H32N2O3 |
| Molar mass | 360.498 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Istaroxime is an investigational drug under development for treatment of acute decompensated heart failure
Originally patented and developed by Sigma-Tau, it was sold to CVie Therapeutics in July 2012.[1]
Heart failure
Istaroxime is a treatment for both systolic and diastolic heart failure.[2]
- Systolic heart failure is characterized by impaired ventricular emptying, caused by reduced contractility.
- Diastolic dysfunction is defined by defective ventricular filling, caused by the heart's inability to properly relax between beats.[3]
Intracellular calcium fluxes regulate both contraction and relaxation. Cardiac muscle cells from patients with heart failure show smaller amounts of peak calcium in their cytoplasm during contraction, and slower removal.,[4][5] The mishandling of intracellular calcium is often due to problems in the cells’ ability to mediate calcium influx, and sequestration of calcium back in the sarcoplasmic reticulum.,[4][6]
Mechanism of action
Istaroxime is a positive inotropic agent[2] that mediates its action through inhibition of sodium/potassium adenosine triphosphatase (Na+/K+ ATPase).[7] Na+/K+ ATPase inhibition increases intracellular sodium levels, which reverses the driving force of the sodium/calcium exchanger, inhibiting calcium extrusion and possibly facilitating calcium entry.,[5][8]
Additionally, istaroxime increases intracellular calcium by improving the efficacy by which intracellular calcium triggers sarcoplasmic reticulum calcium release,[5][8] and by accelerating the inactivation state of L-type calcium channels, which allow for calcium influx.[9] Together the changes in calcium handling increase cell contraction.
Istaroxime also enhances the heart's relaxation phase[5] by increasing the rate of intracellular calcium sequestration by Sarco/endoplasmic Reticulum Calcium ATPase, isotype 2a (SERCA2a).[8] SERCa2a is inhibited by phospholamban and higher phospholamban-to-SERCA2a ratios cause SERCA inhibition and impaired relaxation.[5] Istaroxime reduces SERCA2a-phospholamban interaction,[5][8] and increases SERCA2a affinity for cytosolic calcium.[7] Studies on failing human heart tissue show that istaroxime increases SERCA2a activity up to 67%.[5]
Clinical use
Clinical trials show that istaroxime improves ejection fraction, stroke volume and systolic blood pressure, while also enhancing ventricular filling.[1] The drug also reduces heart rate and ventricular diastolic stiffness.[1] Contrary to available inotropic therapies, istaroxime may permit cytosolic calcium accumulation while avoiding a proarrhythmic state.[5][9][10][11]
Proposed mechanisms for istaroxime's antiarrhythmic effect include a suppression of the transient inward calcium current directly involved in the production of delayed after-depolarizations[5] and improved calcium sequestration due to SERCA2a stimulation.[11] SERCA down-regulation in the failing myocardium[12] might sensitize patients to the detrimental effect of other currently used positive inotropes. Istaroxime's lusitropic effect facilitates its wider margin of safety, as patients can receive higher doses without signs of arrhythmias.[10]
References
- 1 2 3 Shah SJ, Blair JE, Filippatos GS, Macarie C, Ruzyllo W, Korewicki J, et al. (June 2009). "Effects of istaroxime on diastolic stiffness in acute heart failure syndromes: results from the Hemodynamic, Echocardiographic, and Neurohormonal Effects of Istaroxime, a Novel Intravenous Inotropic and Lusitropic Agent: a Randomized Controlled Trial in Patients Hospitalized with Heart Failure (HORIZON-HF) trial". American Heart Journal. 157 (6): 1035–41. doi:10.1016/j.ahj.2009.03.007. PMID 19464414.
- 1 2 Mattera GG, Lo Giudice P, Loi FM, Vanoli E, Gagnol JP, Borsini F, Carminati P (January 2007). "Istaroxime: a new luso-inotropic agent for heart failure". The American Journal of Cardiology. 99 (2A): 33A–40A. doi:10.1016/j.amjcard.2006.09.004. PMID 17239702.
- ↑ Gheorghiade M, Ambrosy AP, Ferrandi M, Ferrari P (August 2011). "Combining SERCA2a activation and Na-K ATPase inhibition: a promising new approach to managing acute heart failure syndromes with low cardiac output". Discovery Medicine. 12 (63): 141–51. PMID 21878191.
- 1 2 Davies CH, Davia K, Bennett JG, Pepper JR, Poole-Wilson PA, Harding SE (November 1995). "Reduced contraction and altered frequency response of isolated ventricular myocytes from patients with heart failure". Circulation. 92 (9): 2540–9. doi:10.1161/01.cir.92.9.2540. PMID 7586355.
- 1 2 3 4 5 6 7 8 9 Micheletti R, Palazzo F, Barassi P, Giacalone G, Ferrandi M, Schiavone A, et al. (January 2007). "Istaroxime, a stimulator of sarcoplasmic reticulum calcium adenosine triphosphatase isoform 2a activity, as a novel therapeutic approach to heart failure". The American Journal of Cardiology. 99 (2A): 24A–32A. doi:10.1016/j.amjcard.2006.09.003. PMID 17239701.
- ↑ Lehnart SE, Schillinger W, Pieske B, Prestle J, Just H, Hasenfuss G (September 1998). "Sarcoplasmic reticulum proteins in heart failure". Annals of the New York Academy of Sciences. 853 (1): 220–30. Bibcode:1998NYASA.853..220L. doi:10.1111/j.1749-6632.1998.tb08270.x. PMID 10603950. S2CID 22792584.
- 1 2 Rocchetti M, Besana A, Mostacciuolo G, Micheletti R, Ferrari P, Sarkozi S, et al. (April 2005). "Modulation of sarcoplasmic reticulum function by Na+/K+ pump inhibitors with different toxicity: digoxin and PST2744 [(E,Z)-3-((2-aminoethoxy)imino)androstane-6,17-dione hydrochloride]". The Journal of Pharmacology and Experimental Therapeutics. 313 (1): 207–15. doi:10.1124/jpet.104.077933. PMID 15576469. S2CID 19954351.
- 1 2 3 4 Rocchetti M, Alemanni M, Mostacciuolo G, Barassi P, Altomare C, Chisci R, et al. (September 2008). "Modulation of sarcoplasmic reticulum function by PST2744 [istaroxime; (E,Z)-3-((2-aminoethoxy)imino) androstane-6,17-dione hydrochloride)] in a pressure-overload heart failure model". The Journal of Pharmacology and Experimental Therapeutics. 326 (3): 957–65. doi:10.1124/jpet.108.138701. PMID 18539651. S2CID 2420808.
- 1 2 Rocchetti M, Besana A, Mostacciuolo G, Ferrari P, Micheletti R, Zaza A (May 2003). "Diverse toxicity associated with cardiac Na+/K+ pump inhibition: evaluation of electrophysiological mechanisms". The Journal of Pharmacology and Experimental Therapeutics. 305 (2): 765–71. doi:10.1124/jpet.102.047696. PMID 12606646. S2CID 116370.
- 1 2 Adamson PB, Vanoli E, Mattera GG, Germany R, Gagnol JP, Carminati P, Schwartz PJ (August 2003). "Hemodynamic effects of a new inotropic compound, PST-2744, in dogs with chronic ischemic heart failure". Journal of Cardiovascular Pharmacology. 42 (2): 169–73. doi:10.1097/00005344-200308000-00003. PMID 12883318. S2CID 25796174.
- 1 2 Alemanni M, Rocchetti M, Re D, Zaza A (May 2011). "Role and mechanism of subcellular Ca2+ distribution in the action of two inotropic agents with different toxicity". Journal of Molecular and Cellular Cardiology. 50 (5): 910–8. doi:10.1016/j.yjmcc.2011.02.008. PMID 21354172.
- ↑ Movsesian MA, Karimi M, Green K, Jones LR (August 1994). "Ca(2+)-transporting ATPase, phospholamban, and calsequestrin levels in nonfailing and failing human myocardium". Circulation. 90 (2): 653–7. doi:10.1161/01.cir.90.2.653. PMID 8044934.