 | |
| Names | |
|---|---|
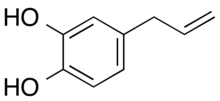
| Preferred IUPAC name
4-(Prop-2-en-1-yl)benzene-1,2-diol | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.208.658 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H10O2 | |
| Molar mass | 150.177 g·mol−1 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H312, H315, H319 | |
| P264, P270, P280, P301+P312, P302+P352, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Hydroxychavicol is a phenylpropanoid compound present in leaves of Piper betle.[1] It is a more potent inhibitor of xanthine oxidase (IC50=16.7 µM) than allopurinol.[2][3]
Research
It might be a useful new compound in treating cutaneous fungal infections.[4] It is a promising agent in prevention and treatment of dental disorders as it had bactericidal and fungicidal effect on Streptococcus intermedius, Streptococcus mutans, and Candida albicans and inhibited biofilm formation.[5][6][7][8]
See also
References
- ↑ Atiya A, Sinha BN, Lal UR (March 2020). "The new ether derivative of phenylpropanoid and bioactivity was investigated from the leaves of Piper betle L". Natural Product Research. 34 (5): 638–645. doi:10.1080/14786419.2018.1495634. PMID 30169967. S2CID 52139286.
- ↑ Murata K, Nakao K, Hirata N, Namba K, Nomi T, Kitamura Y, Moriyama K, Shintani T, Iinuma M, Matsuda H (July 2009). "Hydroxychavicol: a potent xanthine oxidase inhibitor obtained from the leaves of betel, Piper betle". Journal of Natural Medicines. 63 (3): 355–9. doi:10.1007/s11418-009-0331-y. PMID 19387769. S2CID 19647900.
- ↑ Nishiwaki K, Ohigashi K, Deguchi T, Murata K, Nakamura S, Matsuda H, Nakanishi I (July 2018). "Structure-Activity Relationships and Docking Studies of Hydroxychavicol and Its Analogs as Xanthine Oxidase Inhibitors". Chemical & Pharmaceutical Bulletin. 66 (7): 741–747. doi:10.1248/cpb.c18-00197. PMID 29695658.
- ↑ Ali I, Satti NK, Dutt P, Prasad R, Khan IA (November 2016). "Hydroxychavicol: A phytochemical targeting cutaneous fungal infections". Scientific Reports. 6: 37867. Bibcode:2016NatSR...637867A. doi:10.1038/srep37867. PMC 5126685. PMID 27897199.
- ↑ Phumat P, Khongkhunthian S, Wanachantararak P, Okonogi S (May 2020). "Comparative inhibitory effects of 4-allylpyrocatechol isolated from Piper betle on Streptococcus intermedius, Streptococcus mutans, and Candida albicans". Archives of Oral Biology. 113: 104690. doi:10.1016/j.archoralbio.2020.104690. PMID 32155466. S2CID 212664434.
- ↑ Ali, Intzar; Khan, Farrah G; Suri, Krishan A; Gupta, Bishan D; Satti, Naresh K; Dutt, Prabhu; Afrin, Farhat; Qazi, Ghulam N; Khan, Inshad A (2010). "In vitro antifungal activity of hydroxychavicol isolated from Piper betle L". Annals of Clinical Microbiology and Antimicrobials. 9 (1): 7. doi:10.1186/1476-0711-9-7. ISSN 1476-0711. PMC 2841090. PMID 20128889.
- ↑ Himratul-Aznita, Wan Harun; Nor-Zulaila, Che Omran; Nurul-Fatihah, Khairuddin (2016). "Antifungal activity of dual combination of hydroxychavicol with commercialized agents against oral Candida species". SpringerPlus. 5 (1): 1696. doi:10.1186/s40064-016-3396-6. ISSN 2193-1801. PMC 5047859. PMID 27757368.
- ↑ Mail, Mohd Hafiz; Himratul-Aznita, Wan Harun; Musa, Md Yusoff (2017). "Anti-hyphal properties of potential bioactive compounds for oral rinse in suppression of Candida growth". Biotechnology & Biotechnological Equipment. 31 (5): 989–999. doi:10.1080/13102818.2017.1348255. ISSN 1310-2818.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.