| Gurmarin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Structure of gurmarin, a sweet taste-suppressing polypeptide.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Gurmarin | ||||||||
| Pfam | PF11410 | ||||||||
| InterPro | IPR010485 | ||||||||
| SCOP2 | 1gur / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 112 | ||||||||
| OPM protein | 1c4e | ||||||||
| |||||||||
| Gurmarin | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | ? | ||||||
| PDB | 1c4e | ||||||
| UniProt | P25810 | ||||||
| |||||||
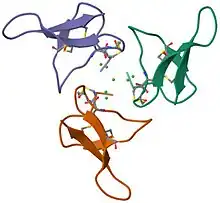
Gurmarin is a 35-residue polypeptide from the Asclepiad vine Gymnema sylvestre (Gurmar). It has been utilized as a pharmacological tool in the study of sweet-taste transduction because of its ability to selectively inhibit the neural response to sweet taste in rats.[1] This rat inhibition appears to have high specificity to sugar (sweetener) molecules like sucrose, glucose, and saccharin as well as the amino acid glycine.[3] As a sweet-taste-suppressing protein, Gurmarin shows signs of being reversible in nature although having little to no effect on the sweet taste sensation in humans suggesting the protein is only active on rodent sweet taste receptors.[4]
Structure and inhibitory effect
Gurmarin is a peptide used to eliminate sweet taste through electrophysiological conditions. It is different from other substances such as gymnemic acid, ziziphin, and hodulcin. This is because these substances are known to suppress sweet taste sensation.[5] It consists of 35 amino acid units and 3 disulfide linkages. It is a member of inhibitor cystine knot peptides (ICK), which are highly stable proteins formed by three disulfide bonds. These ICK peptides are renowned for their remarkable stability, primarily due to the presence of three disulfide bonds. They are not exclusive to only gurmarin, they have also been discovered in a wide variety of organisms, including plants, fungi, vertebrates, and arthropods.[6]
Gurmarin has an inhibitory effect that is found to be reversible, but it is found that there are several hours necessary for recovery of sweet tastes which impairs the perception of sweetness. It is found that beta-cyclodextrin solution can effectively remove gurmarin from the taste tissue.[7] Delving deeper into the molecular interplay of taste, sucralose, a sweet taste molecule emerges as a key player in blocking the activation of sweet taste receptors. Gurmarin plays a role in reducing sucralose induced elevation in cytosolic calcium levels. It also plays a crucial role in glucose sensing. Approximately 70% of glucose-excited neurons in the arcuate nucleus are inhibited by gurmarin. This demonstrates that gurmarin has the ability to inhibit the activation of the receptor, which therefore affects the impact on feeding behavior and the maintenance of energy.[8]
Impact on umami taste
Although gurmarin's inhibitory action is highly specific to sweet taste, it also impacts umami taste. Umami taste usually contains high levels of amino acid glutamate. This suggests that there is a relationship between the two, sweet and umami taste. It is found that the interaction site of gurmarin is the apical membrane of taste cells, which is notably influenced by the pH environment.[9] In rats, it was found that gurmarin suppressed the response of the chorda tympani (CT) nerve in rats, but it did not affect the responses of the glossopharyngeal (GL) nerve to any tastes that were tested which included umami substances (US).[10]
Influence on the central nervous system
Gurmarin influences the central nervous system. Many nuclei of the solitary tract neurons were found to be highly sensitive to gurmarin. This indicates that there are multiple receptor mechanisms that allow for sweet taste in the central nervous system. Some solitary tract neurons had exhibited the ability to continuously respond to the gurmarin inhibitory effects of sweet taste, even after treatment. This raises the suggestion that there is synaptic coupling among the taste receptor cells driven by the gurmarin-sensitive and -insensitive receptor mechanisms.[11]
Diabetic and therapeutic effects
Gurmarin can play a role in the field of managing diabetes. It is one of many active compounds that are of high interest as an antidiabetic. As mentioned, it is derived from Gurmar. Gurmar represents a positive contribution to blood sugar and has the ability to control the cravings of sugar. This means it ultimately reduces the amount of sugar intake. Gurmarin is one of many active compounds found through the insulin secretion from beta cells in the pancreas.[12]
As a herb, gurmarin being a polypeptide of Gymnema sylvestre, it has a broad range of therapeutic effects for other health conditions. This encompasses conditions such as arthritis, diuretic properties, anemia, osteoporosis, high cholesterol levels, heart conditions, asthma, digestive discomfort, microbial infections, indigestion, and concerns related to inflammation. It even has shown as a benefit to maintaining weight and lowering both blood cholesterol levels and triglyceride concentrations.[13]
References
- 1 2 Arai K, Ishima R, Morikawa S, Miyasaka A, Imoto T, Yoshimura S, et al. (April 1995). "Three-dimensional structure of gurmarin, a sweet taste-suppressing polypeptide". Journal of Biomolecular NMR. 5 (3): 297–305. doi:10.1007/BF00211756. PMID 7787425. S2CID 36794097.
- ↑ "Crystal structure of gurmarin, a sweet taste suppressing polypeptide". RCSB Protein Data Bank. 5OLL. Retrieved 2023-11-28.
- ↑ "Gurmarin GUR_GYMSY". Uniprot.org. May 1, 1992. P25810. Retrieved May 16, 2022.
- ↑ Sigoillot M, Brockhoff A, Meyerhof W, Briand L (November 2012). "Sweet-taste-suppressing compounds: current knowledge and perspectives of application". Applied Microbiology and Biotechnology. 96 (3): 619–630. doi:10.1007/s00253-012-4387-3. PMID 22983596.
- ↑ Kurihara Y, Nirasawa S (February 1994). "Sweet, antisweet and sweetness-inducing substances". Trends in Food Science & Technology. 5 (2): 37–42. doi:10.1016/0924-2244(94)90069-8. ISSN 0924-2244.
- ↑ Sigoillot M, Brockhoff A, Neiers F, Poirier N, Belloir C, Legrand P, et al. (September 2018). "The Crystal Structure of Gurmarin, a Sweet Taste-Suppressing Protein: Identification of the Amino Acid Residues Essential for Inhibition". Chemical Senses. 43 (8): 635–643. doi:10.1093/chemse/bjy054. PMID 30137256.
- ↑ Sigoillot M, Brockhoff A, Meyerhof W, Briand L (November 2012). "Sweet-taste-suppressing compounds: current knowledge and perspectives of application". Applied Microbiology and Biotechnology. 96 (3): 619–630. doi:10.1007/s00253-012-4387-3. PMID 22983596.
- ↑ Kohno D (July 2017). "Sweet taste receptor in the hypothalamus: a potential new player in glucose sensing in the hypothalamus". The Journal of Physiological Sciences. 67 (4): 459–465. doi:10.1007/s12576-017-0535-y. PMC 10717116. PMID 28378265.
- ↑ Miyasaka A, Imoto T (April 1995). "Electrophysiological characterization of the inhibitory effect of a novel peptide gurmarin on the sweet taste response in rats". Brain Research. 676 (1): 63–68. doi:10.1016/0006-8993(95)00086-6. PMID 7796179.
- ↑ Ninomiya Y, Nakashima K, Fukuda A, Nishino H, Sugimura T, Hino A, et al. (April 2000). "Responses to umami substances in taste bud cells innervated by the chorda tympani and glossopharyngeal nerves". The Journal of Nutrition. 130 (4S Suppl): 950S–953S. doi:10.1093/jn/130.4.950S. PMID 10736359.
- ↑ Lemon CH, Imoto T, Smith DV (August 2003). "Differential gurmarin suppression of sweet taste responses in rat solitary nucleus neurons". Journal of Neurophysiology. 90 (2): 911–923. doi:10.1152/jn.00215.2003. PMID 12702710.
- ↑ Ríos JL, Francini F, Schinella GR (August 2015). "Natural Products for the Treatment of Type 2 Diabetes Mellitus". Planta Medica. 81 (12–13): 975–994. doi:10.1055/s-0035-1546131. hdl:11336/49479. PMID 26132858.
- ↑ Tiwari P, Mishra BN, Sangwan NS (2014-01-06). "Phytochemical and pharmacological properties of Gymnema sylvestre: an important medicinal plant". BioMed Research International. 2014: 830285. doi:10.1155/2014/830285. PMC 3912882. PMID 24511547.