 | |
| Names | |
|---|---|
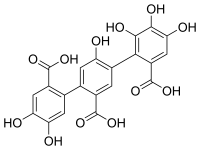
| Preferred IUPAC name
14,15,25,34,35,36-Hexahydroxy[11,21:24,31-terphenyl]-12,22,32-tricarboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C21H14O12 | |
| Molar mass | 458.32 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Flavogallonic acid is a hydrolysable tannin that can be found in valonea oak (Quercus macrolepis)[1] in chestnut wood[2] or in Terminalia myriocarpa.[3]
See also
References
- ↑ Molecular investigation of valonea tannin. Hasan Özgunay and Özcan Sari, The Journal of the American Leather Chemists Association, 2007, vol. 102, no5, pp. 154-157, INIST 18744048, text
- ↑ Considerations on the macromolecular structure of chestnut ellagitannins by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Pasch H and Pizzi A, Journal of applied polymer science, 2002, vol. 85, no2, pp. 429-437, INIST 14185517
- ↑ Pharmacologically Active Ellagitannins from Terminalia myriocarpa. Mohamed S.A. Marzouk, Sayed A.A. El-Toumy, Fatma A. Moharram, Nagwa M.M. Shalaby and Amany A.E. Ahmed, Planta Med, 2002, 68(6), pages 523-527, doi:10.1055/s-2002-32549
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.