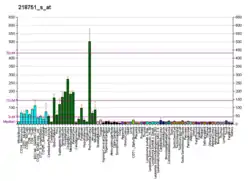
F-box/WD repeat-containing protein 7 is a protein that in humans is encoded by the FBXW7 gene.[5][6][7]
Function
This gene encodes a member of the F-box protein family which is characterized by an approximately 40 amino acid motif, the F-box. The F-box proteins constitute one of the four subunits of ubiquitin protein ligase complex called SCFs (SKP1-cullin-F-box), which function in phosphorylation-dependent ubiquitination. The F-box proteins are divided into 3 classes: Fbws containing WD-40 domains, Fbls containing leucine-rich repeats, and Fbxs containing either different protein-protein interaction modules or no recognizable motifs. The protein encoded by this gene was previously referred to as FBX30, and belongs to the Fbws class; in addition to an F-box, this protein contains 7 tandem WD40 repeats. This protein binds directly to cyclin E and probably targets cyclin E for ubiquitin-mediated degradation. Other well established pro-proliferative targets of FBXW7 are c-Myc and Notch1. Mono-allelic mutations in this gene are detected in sporadic cancers [e.g., cholangiocarcinoma (35%), T-ALL (31%), endometrial carcinoma (16%), colorectal carcinoma (16%), bladder cancer (10%), gastric carcinoma (6%), lung squamous cell carcinoma (5%), etc.]. These findings implicate the gene's potential role in the pathogenesis of human cancers. Despite being commonly acknowledged as a haploinsufficient tumor suppressor, mutations are not found in some cancers, such as acute myeloid leukemia and multiple myeloma. One possibility is that FBXW7 substrate stabilization is detrimental in these neoplasms. For example, the FBXW7 substrate C/EBPα suppresses AML[8] and multiple myelomas require constitutive NF-κB signaling; therefore, disruption of FBXW7-mediated ubiquitylation of IκBd in these tumors results in cell death.[9][10]
Three transcript variants encoding three different isoforms have been found for this gene.[7]
Interactions
FBXW7 has been shown to interact with:
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000109670 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000028086 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Winston JT, Koepp DM, Zhu C, Elledge SJ, Harper JW (Dec 1999). "A family of mammalian F-box proteins". Curr Biol. 9 (20): 1180–2. doi:10.1016/S0960-9822(00)80021-4. PMID 10531037. S2CID 14341845.
- ↑ Gupta-Rossi N, Le Bail O, Gonen H, Brou C, Logeat F, Six E, Ciechanover A, Israël A (Sep 2001). "Functional interaction between SEL-10, an F-box protein, and the nuclear form of activated Notch1 receptor". J Biol Chem. 276 (37): 34371–8. doi:10.1074/jbc.M101343200. PMID 11425854.
- 1 2 "Entrez Gene: FBXW7 F-box and WD repeat domain containing 7".
- ↑ Bengoechea-Alonso MT, Ericsson J (June 2010). "The ubiquitin ligase Fbxw7 controls adipocyte differentiation by targeting C/EBPalpha for degradation". Proceedings of the National Academy of Sciences of the United States of America. 107 (26): 11817–22. Bibcode:2010PNAS..10711817B. doi:10.1073/pnas.0913367107. PMC 2900639. PMID 20534483.
- ↑ Busino L, Millman SE, Scotto L, Kyratsous CA, Basrur V, O'Connor O, Hoffmann A, Elenitoba-Johnson KS, Pagano M (March 2012). "Fbxw7α- and GSK3-mediated degradation of p100 is a pro-survival mechanism in multiple myeloma". Nature Cell Biology. 14 (4): 375–85. doi:10.1038/ncb2463. PMC 3339029. PMID 22388891.
- ↑ Busino L, Millman SE, Pagano M (December 2012). "SCF-mediated degradation of p100 (NF-κB2): mechanisms and relevance in multiple myeloma". Science Signaling. 5 (253): pt14. doi:10.1126/scisignal.2003408. PMC 3871187. PMID 23211527.
- ↑ Kanei-Ishii C, Nomura T, Takagi T, Watanabe N, Nakayama KI, Ishii S (Nov 2008). "Fbxw7 acts as an E3 ubiquitin ligase that targets c-Myb for nemo-like kinase (NLK)-induced degradation". J. Biol. Chem. 283 (45): 30540–8. doi:10.1074/jbc.M804340200. PMC 2662147. PMID 18765672.
- ↑ Olson BL, Hock MB, Ekholm-Reed S, Wohlschlegel JA, Dev KK, Kralli A, Reed SI (Jan 2008). "SCFCdc4 acts antagonistically to the PGC-1alpha transcriptional coactivator by targeting it for ubiquitin-mediated proteolysis". Genes Dev. 22 (2): 252–64. doi:10.1101/gad.1624208. PMC 2192758. PMID 18198341.
- 1 2 Staropoli JF, McDermott C, Martinat C, Schulman B, Demireva E, Abeliovich A (Mar 2003). "Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity". Neuron. 37 (5): 735–49. doi:10.1016/s0896-6273(03)00084-9. PMID 12628165. S2CID 17024826.
- ↑ Wu G, Lyapina S, Das I, Li J, Gurney M, Pauley A, Chui I, Deshaies RJ, Kitajewski J (Nov 2001). "SEL-10 is an inhibitor of notch signaling that targets notch for ubiquitin-mediated protein degradation". Mol. Cell. Biol. 21 (21): 7403–15. doi:10.1128/MCB.21.21.7403-7415.2001. PMC 99913. PMID 11585921.
Further reading
- Robertson NG, Khetarpal U, Gutiérrez-Espeleta GA, Bieber FR, Morton CC (1995). "Isolation of novel and known genes from a human fetal cochlear cDNA library using subtractive hybridization and differential screening". Genomics. 23 (1): 42–50. doi:10.1006/geno.1994.1457. PMID 7829101.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Oberg C, Li J, Pauley A, Wolf E, Gurney M, Lendahl U (2001). "The Notch intracellular domain is ubiquitinated and negatively regulated by the mammalian Sel-10 homolog". J. Biol. Chem. 276 (38): 35847–53. doi:10.1074/jbc.M103992200. PMID 11461910.
- Koepp DM, Schaefer LK, Ye X, Keyomarsi K, Chu C, Harper JW, Elledge SJ (2001). "Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase". Science. 294 (5540): 173–7. Bibcode:2001Sci...294..173K. doi:10.1126/science.1065203. PMID 11533444. S2CID 23404627.
- Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK (2001). "Archipelago regulates Cyclin E levels in Drosophila and is mutated in human cancer cell lines". Nature. 413 (6853): 311–6. Bibcode:2001Natur.413..311M. doi:10.1038/35095068. PMID 11565033. S2CID 4372821.
- Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI (2001). "Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line". Nature. 413 (6853): 316–22. Bibcode:2001Natur.413..316S. doi:10.1038/35095076. PMID 11565034. S2CID 478973.
- Wu G, Lyapina S, Das I, Li J, Gurney M, Pauley A, Chui I, Deshaies RJ, Kitajewski J (2001). "SEL-10 Is an Inhibitor of Notch Signaling That Targets Notch for Ubiquitin-Mediated Protein Degradation". Mol. Cell. Biol. 21 (21): 7403–15. doi:10.1128/MCB.21.21.7403-7415.2001. PMC 99913. PMID 11585921.
- Spruck CH, Strohmaier H, Sangfelt O, Müller HM, Hubalek M, Müller-Holzner E, Marth C, Widschwendter M, Reed SI (2002). "hCDC4 gene mutations in endometrial cancer". Cancer Res. 62 (16): 4535–9. PMID 12183400.
- Li J, Pauley AM, Myers RL, Shuang R, Brashler JR, Yan R, Buhl AE, Ruble C, Gurney ME (2002). "SEL-10 interacts with presenilin 1, facilitates its ubiquitination, and alters A-beta peptide production". J. Neurochem. 82 (6): 1540–8. doi:10.1046/j.1471-4159.2002.01105.x. PMID 12354302. S2CID 29687815.
- Staropoli JF, McDermott C, Martinat C, Schulman B, Demireva E, Abeliovich A (2003). "Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity". Neuron. 37 (5): 735–49. doi:10.1016/S0896-6273(03)00084-9. PMID 12628165. S2CID 17024826.
- Calhoun ES, Jones JB, Ashfaq R, Adsay V, Baker SJ, Valentine V, Hempen PM, Hilgers W, Yeo CJ, Hruban RH, Kern SE (2003). "BRAF and FBXW7 (CDC4, FBW7, AGO, SEL10) Mutations in Distinct Subsets of Pancreatic Cancer: Potential Therapeutic Targets". Am. J. Pathol. 163 (4): 1255–60. doi:10.1016/S0002-9440(10)63485-2. PMC 1868306. PMID 14507635.
- Welcker M, Singer J, Loeb KR, Grim J, Bloecher A, Gurien-West M, Clurman BE, Roberts JM (2003). "Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation". Mol. Cell. 12 (2): 381–92. doi:10.1016/S1097-2765(03)00287-9. PMID 14536078.
- Busino L, Donzelli M, Chiesa M, Guardavaccaro D, Ganoth D, Dorrello NV, Hershko A, Pagano M, Draetta GF (2003). "Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage". Nature. 426 (6962): 87–91. Bibcode:2003Natur.426...87B. doi:10.1038/nature02082. PMID 14603323. S2CID 768783.
- Cassia R, Moreno-Bueno G, Rodríguez-Perales S, Hardisson D, Cigudosa JC, Palacios J (2004). "Cyclin E gene (CCNE) amplification and hCDC4 mutations in endometrial carcinoma". J. Pathol. 201 (4): 589–95. doi:10.1002/path.1474. PMID 14648662. S2CID 26390858.
- Nateri AS, Riera-Sans L, Da Costa C, Behrens A (2004). "The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling". Science. 303 (5662): 1374–8. Bibcode:2004Sci...303.1374N. doi:10.1126/science.1092880. PMID 14739463. S2CID 36368075.