 | |
 | |
| Names | |
|---|---|
| Other names
Vescalagin (treatment) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C41H26O26 | |
| Molar mass | 934.63 g/mol |
| Appearance | off-white amorphous powder [2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
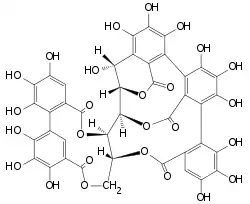
Castalagin is an ellagitannin, a type of hydrolyzable tannin, found in oak and chestnut wood[3] and in the stem barks of Anogeissus leiocarpus and Terminalia avicennoides.[4]
Castalagin is the diastereomer of vescalagin in C-1 of the glycosidic chain.[5] Castalagin/ vescalagin are the most abundant ellagitannins in white wine stored in oak barrels.[6] During aging of wines, these two compounds were progressively extracted from the wood and were transformed into new derivatives by chemical reactions.[7] Therefore, castalagin/ vescalagin and their derivatives contribute to the color and the taste of wines and spirits stored in oak barrels. [5]
Sources
Castalagin was first isolated in Fagaceae family woody species : Quercus (oak) and Castanea (chestnut) by Walter Mayer and co-workers (1967). [8] In some chestnut species, such as Castanea sativa, heartwood could contain 63 mg of castalagin/ vescalagin per gram of dry wood. [9] In some wines, these two isomers represent about 40 to 70% of total ellagitannins. [10]
Biosynthesis
In some plants including oak and chestnut, the ellagitannins are formed from 1,2,3,4,6-pentagalloyl-glucose and further elaborated via oxidative dehydrogenation (tellimagrandin II and casuarictin formations). After conversion of casuarictin to pedunculagin, the pyranose ring of the glucose opens and the family of compounds including casuariin, casuarinin, castalagin, and castlin, vescalagin and vescalin forms.[11]
Castalagin thus forms from a pentagalloyl-glucose structure. Castalagin and vescalagin (1,2,3,5-nonahydroxytriphenoyl-4,6-hexahydroxydiphenoyl-glucoses) can be further polymerized in their corresponding dimers roburin A[12] and roburin D, and 33-carboxy-33-deoxyvescalagin.[13]
Derivatives
Castalagin and other related ellagitanins polymerizes or forms complexes with anthyocyanins and flavonoids. The flavono-ellagitannin known as acutissimin A is created when the oak tannin vescalagin interacts with catechin a flavan-3-ol found in wine. [14] Grandinin is a castalagin glycoside [15] which forms by binding to the pentose lyxose.[16] Chemical hydrolysis of Castalagin/ Vescalagin produces vescalene and vescalin which are potent topoisomerase II inhibitors. [17]
References
- ↑ "Castalagin". PubChem. 2017-07-29.
- ↑ Yannai, Shmuel (2012-10-23). Dictionary of Food Compounds with CD-ROM, Second Edition. CRC Press. p. 861. ISBN 978-1-4200-8351-4.
- ↑ Zahri, S; Belloncle, C; Charrier, F; Pardon, P; Quideau, S; Charrier, B (2007). "UV light impact on ellagitannins and wood surface colour of European oak (Quercus petraea and Quercus robur)". Applied Surface Science. 253 (11): 4985–9. doi:10.1016/j.apsusc.2006.11.005.
- ↑ Shuaibu MN, Pandey K, Wuyep PA, et al. (November 2008). "Castalagin from Anogeissus leiocarpus mediates the killing of Leishmania in vitro". Parasitology Research. 103 (6): 1333–8. doi:10.1007/s00436-008-1137-7. PMID 18690475. S2CID 37480828.
- 1 2 Vivas N, Laguerre M, Pianet de Boissel I, Vivas de Gaulejac N, Nonier MF (April 2004). "Conformational interpretation of vescalagin and castalagin physicochemical properties". Journal of Agricultural and Food Chemistry. 52 (7): 2073–8. doi:10.1021/jf030460m. PMID 15053554.
- ↑ Marinov, M. G.; Dimitrova, E. D.; Puech, J. -L. (1997). "Kinetics of ellagitannin extraction from oak wood using white wine". Journal of Wine Research. 8: 29–40. doi:10.1080/09571269708718095.
- ↑ Puech JL, Mertz C, Michon V, Le Guernevé C, Doco T, Hervé Du Penhoat C (May 1999). "Evolution of castalagin and vescalagin in ethanol solutions. Identification of new derivatives". Journal of Agricultural and Food Chemistry. 47 (5): 2060–6. doi:10.1021/jf9813586. PMID 10552496.
- ↑ Mayer, Walter; Gabler, Wilfried; Riester, Alfons; Korger, Helfried (1967). "Über die Gerbstoffe aus dem Holz der Edelkastanie und der Eiche, II. Die Isolierung von Castalagin, Vescalagin, Castalin und Vescalin". Justus Liebigs Annalen der Chemie (in German). 707 (1): 177–181. doi:10.1002/jlac.19677070125. ISSN 1099-0690.
- ↑ Viriot, Carole; Scalbert, Augustin; Hervé Du Penhoat, Catherine L.M.; Moutounet, Michel (1994-08-10). "Ellagitannins in woods of sessile oak and sweet chestnut dimerization and hydrolysis during wood ageing". Phytochemistry. 36 (5): 1253–1260. doi:10.1016/S0031-9422(00)89647-8. ISSN 0031-9422.
- ↑ Gadrat, Mathilde; Lavergne, Joel; Emo, Catherine; Teissedre, Pierre-Louis; Chira, Kleopatra (2021-02-10). "Ellagitannins quantification in oak wood and cognac eaux-de-vie: Sourced from the research article "Validation of a Mass Spectrometry Method to Identify and Quantify Ellagitannins in Oak Wood and Cognac during Aging in Oak Barrels." (Food Chem., 2020). Original language of the article: English". IVES Technical Reviews, Vine and Wine. doi:10.20870/IVES-TR.2021.4610. ISSN 2680-4905.
- ↑ Quideau, Stephane (2009-01-05). Chemistry And Biology Of Ellagitannins: An Underestimated Class Of Bioactive Plant Polyphenols. World Scientific. pp. 327–333. ISBN 978-981-4471-44-2.
- ↑ Herve Du Penhoat, Catherine L.M.; Michon, Veronique M.F.; Ohassan, Abdelhamid; Peng, Shuyun; Scalbert, Augustin; Gage, Douglas (1991). "Roburin A, A dimeric ellagitannin from heartwood of Quercus robur". Phytochemistry. 30: 329–32. doi:10.1016/0031-9422(91)84148-L.
- ↑ Glabasnia, Arne; Hofmann, Thomas (2007). "Identification and Sensory Evaluation of Dehydro- and Deoxy-ellagitannins Formed upon Toasting of Oak Wood (Quercus alba L.)". Journal of Agricultural and Food Chemistry. 55 (10): 4109–18. doi:10.1021/jf070151m. PMID 17444655.
- ↑ Gil, Luís; Pereira, Carlos; Branco, P.; Teixeira, Artur (2006-12-31). "Formation of acutissimin A in red wine through the contact with cork". OENO One. 40 (4): 217–222. doi:10.20870/oeno-one.2006.40.4.862. ISSN 2494-1271.
- ↑ Fridrich, Diana; Glabasnia, Arne; Fritz, Jessica; Esselen, Melanie; Pahlke, Gudrun; Hofmann, Thomas; Marko, Doris (2008). "Oak Ellagitannins Suppress the Phosphorylation of the Epidermal Growth Factor Receptor in Human Colon Carcinoma Cells". Journal of Agricultural and Food Chemistry. 56 (9): 3010–15. doi:10.1021/jf073427z. PMID 18419129.
- ↑ Hofmann T, Glabasnia A, Schwarz B, Wisman KN, Gangwer KA, Hagerman AE (December 2006). "Protein Binding and Astringent Taste of a Polymeric Procyanidin, 1,2,3,4,6-Penta-O-galloyl-β-D-glucopyranose, Castalagin and Grandinin". Journal of Agricultural and Food Chemistry. 54 (25): 9503–9. doi:10.1021/jf062272c. PMC 2597504. PMID 17147439.
- ↑ Quideau, Stéphane; Jourdes, Michael; Lefeuvre, Dorothée; Montaudon, Danièle; Saucier, Cédric; Glories, Yves; Pardon, Patrick; Pourquier, Philippe (2005-11-04). "The Chemistry of Wine PolyphenolicC-Glycosidic Ellagitannins Targeting Human Topoisomerase II". Chemistry - A European Journal. 11 (22): 6503–6513. doi:10.1002/chem.200500428. ISSN 0947-6539. PMID 16110520.