Boron neutron capture therapeutics are pharmaceuticals used to deliver boron-10 to cancerous cells as part of boron neutron capture therapy (BNCT). Boron-10 atoms strongly absorb neutrons to form a metastable state of boron-11, which undergoes α-decay. By accumulating boron-10 in cancerous cells and subjecting the tumor to neutron radiation, high-energy α particles are selectively delivered only to the target cells.[1] In order for BNCT to be effective, safe, and successful, therapeutic candidates must be non-toxic, must selectively accumulate in target tissue and not normal tissue, and must remain in target tissue while fading from the blood stream.[2] As of 2023, the technology is available in Japan only, and even there few implementations have been reported.[3]
Initial proposal and development
Neutron capture therapy was first proposed in the literature in 1936 by Gordon L. Locher, who observed that isotopes with large neutron capture cross sections, such as boron-10, could be accumulated in cancerous tissue and bombarded with thermal neutrons to induce destruction of the cancerous cells.[4] This idea was attractive because it had the potential to be more selective than traditional chemo- and radiotherapies.[1] However, BNCT requires a neutron beam to act as a source of thermal neutrons and a suitable boron-delivery agent; neither was available at the time of Locher's suggestion. Therefore, it was not until the 1950s, when nuclear reactors were available to medical researchers, that Locher's proposal was put into practice.[5]
Boron delivery agents
Requirements for boron delivery agents
A BNCT therapeutic candidate must selectively accumulate in target tissue without significant uptake in normal tissue. If selectivity is low and boron accumulates in both, irradiation with thermal neutrons will cause significant damage to healthy tissue; if boron accumulates in neither, the treatment will be ineffective. Selectivity is quantified by the tumor⁄normal tissue boron ratio, which compares the concentration of boron atoms in tumor cells with that in the patient's healthy cells.[6] A large tumor⁄normal tissue ratio (~3 or greater) is necessary.[2] In addition, boron must remain in target tissue at significant concentrations (~20 μg/g) for long enough that concentration in the blood drops to low levels (generally several hours).[6]
Early candidates
Early work in the 1950s made use of widely available non-toxic boron compounds such as sodium borate (also known as borax) and boric acid. Sodium borate was used to treat nearly a dozen patients with BNCT through a collaboration between Massachusetts General Hospital and Brookhaven National Laboratory.[5] The results were inconclusive and lack of success was blamed on the short lifetime of the tumor:normal tissue differential.[6][7]
Seeking to improve selectivity through chemical modification, studies were performed to correlate lipid solubility with penetration of the blood-brain barrier in mice.[10][11] It was determined that compounds with high solubility in benzene are more capable of penetrating the brain, and should thus be avoided as BNCT therapeutics. Based on this data, boron-10 enriched samples of p-carboxyphenylboronic acid (PCPB) and sodium decahydrodecaborate (Na2B10H10) were selected for BNCT at the Massachusetts Institute of Technology research reactor. The therapy was performed on eighteen patients before the realization that patients were receiving severe radiation damage to normal tissue ended the trial.[12] Later analysis established the likely cause of death as radiation necrosis for at least nine patients, and the study has been described as a “total failure”.[7][12] Radiation necrosis was attributed to fission of boron-10 atoms in the bloodstream, damaging adjacent blood vessels.[13] PCPB and Na2B10H10 had been selected for their promising tumor:normal tissue differentials; however, the concentration of boron-10 in patients’ blood was not considered as significant a concern until after these results.[1]
Second-generation boron delivery agents
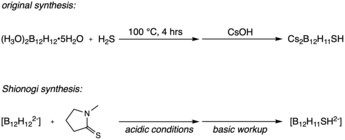
Building on the dodecaborane anion ([B12H12]2-) discovered in part by M. Frederick Hawthorne, Earl Muetterties et al. developed the monosubstituted derivative sodium borocaptate (BSH, Na2B12H11SH).[14][15] While screening boron compounds for use in BNCT, it was discovered that BSH and other monosubstituted sulfhydryl boron hydride clusters accumulate in cancerous cells without lingering in the blood stream — exactly the properties needed for new BNCT therapeutics.[11] It has been theorized that the thiol moiety present in BSH plays a role in the differing biological uptake properties between [B12H12]2- and BSH, though more research must be done to understand the relevant biochemistry.[7][11]
Although clinical work with BNCT in the United States was paused for decades after the MIT reactor experiments, BNCT continued in Japan. Using BSH synthesized by the Shionogi pharmaceutical company, BNCT was used to treat over 200 patients by Hiroshi Hatanaka, Yoshinobu Nakagawa, and their colleagues.[16][17] The success and efficacy of the Hatanaka/Nakagawa work has been debated, with some arguing that there was no significant improvement in patient outcomes; others argue, however, that critics are focusing on small subsamples of the patient population, and are not taking into account that neutron beams accessible to Hatanaka were much less powerful than those at Brookhaven and at MIT.[13][1][18] Further studies have been carried out in the Netherlands and the Czech Republic, and BSH continues to be tested for BNCT.[19][3][20]

First synthesized in 1958 by Snyder et al., boronophenylalanine (BPA) and its more water-soluble fructose complex (BPA-F) were not initially acknowledged as potential BNCT therapeutics.[21] In the 1970s, however, researchers in Japan proposed that BPA could be used to target malignant melanomas with BNCT.[22] Previous work on BNCT had only targeted cancers of the brain, using the blood-brain barrier to improve tumor:normal tissue differentials.[20] Researchers argued, however, that similarities between BPA and the precursor amino acids to melanin meant that melanomas may selectively accumulate BPA. Clinical trials began again in the United States in the 1990s at Brookhaven and at MIT using BPA, both for melanomas and glioblastomas. In contrast with the Japanese BSH trials, higher-energy epithermal neutrons were used in place of thermal neutrons, allowing for deeper penetration into the brain without the need for neurosurgery during treatment.[23] Further trials with BPA have been carried out in Finland, the Netherlands, Sweden, Taiwan, and Japan. In some trials, both BPA and BSH were used as the delivery agent, and some studies have tested the effectiveness of BNCT in conjunction with traditional chemo- and radiotherapies.[24] The primary flaw with both BSH and BPA/BPA-F seems to be heterogeneity in distribution of boron-10 throughout the tumors.[2][23]
Third-generation boron delivery agents
Despite some successes with BSH and BPA, broad variability in tumor uptake and heterogeneous distribution within tumors have led researchers to pursue updated alternatives.[2] Third-generation boron delivery agents are marked by inclusion of a specific chemical tumor-targeting moiety, often borrowed from those established in chemotherapy, linked to a boron-carrying compound. These targeted drug delivery systems are designed to bind the delivery agent to chemical sites found in tumor cells, rather than relying on secondary properties such as hydrophilicity; the use of BPA to target melanomas was an early example.[24] Examples of compounds derivativized for BNCT include "peptides, proteins, antibodies, nucleosides, sugars, porphyrins, liposomes and nanoparticles."[20] While animal and in vitro studies have shown potential, no third-generation boron delivery agent has yet been used in a clinical trial.[6]
References
- 1 2 3 4 Hawthorne, M. Frederick (July 1993). "The Role of Chemistry in the Development of Boron Neutron Capture Therapy of Cancer". Angewandte Chemie International Edition in English. 32 (7): 950–984. doi:10.1002/anie.199309501. ISSN 0570-0833.
- 1 2 3 4 Barth, Rolf F.; Mi, Peng; Yang, Weilian (2018). "Boron delivery agents for neutron capture therapy of cancer". Cancer Communications. 38 (1): 35. doi:10.1186/s40880-018-0299-7. ISSN 2523-3548. PMC 6006782. PMID 29914561.
- 1 2 Malouff, Timothy D.; Seneviratne, Danushka S.; Ebner, Daniel K.; Stross, William C.; Waddle, Mark R.; Trifiletti, Daniel M.; Krishnan, Sunil (2021-02-26). "Boron Neutron Capture Therapy: A Review of Clinical Applications". Frontiers in Oncology. 11. doi:10.3389/fonc.2021.601820. ISSN 2234-943X. PMC 7952987. PMID 33718149.
- ↑ Locher, Gordon L. (July 1936). "Biological effects and therapeutic possibilities of neutrons". The American Journal of Roentgenology and Radium Therapy. 36 (1): 1–13.
- 1 2 Farr, L. E.; Sweet, W. H.; Robertson, J. S.; Foster, C. G.; Locksley, H. B.; Sutherland, D. L.; Mendelsohn, M. L.; Stickley, E. E. (February 1954). "Neutron capture therapy with boron in the treatment of glioblastoma multiforme". The American Journal of Roentgenology, Radium Therapy, and Nuclear Medicine. 71 (2): 279–293. ISSN 0002-9580. PMID 13124616.
- 1 2 3 4 Barth, Rolf F.; Coderre, Jeffrey A.; Vicente, M. Graça H.; Blue, Thomas E. (2005-06-01). "Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects". Clinical Cancer Research. 11 (11): 3987–4002. doi:10.1158/1078-0432.CCR-05-0035. ISSN 1078-0432. PMID 15930333.
- 1 2 3 Soloway, Albert H.; Tjarks, Werner; Barnum, Beverly A.; Rong, Feng-Guang; Barth, Rolf F.; Codogni, Iwona M.; Wilson, J. Gerald (1998-06-01). "The Chemistry of Neutron Capture Therapy". Chemical Reviews. 98 (4): 1515–1562. doi:10.1021/cr941195u. ISSN 0009-2665. PMID 11848941.
- ↑ Hawthorn, M. F.; Pilling, R. L.; Knoth, W. H. (1967), Tyree, S. Y. (ed.), "Bis(triethylammonium) Decahydrodecaborate(2−)", Inorganic Syntheses (1 ed.), Wiley, vol. 9, pp. 16–19, doi:10.1002/9780470132401.ch6, ISBN 978-0-470-13168-8, retrieved 2023-12-20
- ↑ Sweet, W. H.; Soloway, A. H.; Wright, R. L. (1962-08-01). "Evaluation of Boron Compounds for Use in Neutron Capture Therapy of Brain Tumors. Ii. Studies in Man". Journal of Pharmacology and Experimental Therapeutics. 137 (2): 263–266. ISSN 0022-3565.
- ↑ Soloway, A. H. (1958). "Correlation of Drug Penetration of Brain and Chemical Structure". Science. 128 (3338): 1572–1574. doi:10.1126/science.128.3338.1572.b. ISSN 0036-8075. JSTOR 1755566.
- 1 2 3 Soloway, A. H.; Whitman, B.; Messer, J. R. (1960-07-01). "Penetration of Brain and Brain Tumor by Aromatic Compounds as a Function of Molecular Substituents". Journal of Pharmacology and Experimental Therapeutics. 129 (3): 310–314. ISSN 0022-3565. PMID 13832708.
- 1 2 Asbury, Arthur K.; Ojemann, Robert G.; Nielsen, Surl L.; Sweet, William H. (1972). "Neuropathologic Study of Fourteen Cases of Malignant Brain Tumor Treated by Boron-10 Slow Neutron Capture Radiation". Journal of Neuropathology and Experimental Neurology. 31 (2): 278–303. doi:10.1097/00005072-197204000-00005. ISSN 0022-3069. PMID 4337274. S2CID 26819849.
- 1 2 Sweet, William H. (1997). "Early History of Development of Boron Neutron Capture Therapy of Tumors". Journal of Neuro-Oncology. 33 (1/2): 19–26. doi:10.1023/A:1005752827194. PMID 9151220. S2CID 21034043.
- ↑ Pitochelli, Anthony R.; Hawthorne, Frederick M. (1960). "THE ISOLATION OF THE ICOSAHEDRAL B12H12-2 ION". Journal of the American Chemical Society. 82 (12): 3228–3229. doi:10.1021/ja01497a069. ISSN 0002-7863.
- ↑ Miller, H. C.; Miller, N. E.; Muetterties, E. L. (1964). "Chemistry of Boranes. XX. Syntheses of Polyhedral Boranes". Inorganic Chemistry. 3 (10): 1456–1463. doi:10.1021/ic50020a026. ISSN 0020-1669.
- ↑ Hatanaka, H. (1991), Karim, A. B. M. F.; Laws, Edward R. (eds.), "Boron-Neutron Capture Therapy for Tumors", Glioma, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 233–249, doi:10.1007/978-3-642-84127-9_18, ISBN 978-3-540-52286-7, retrieved 2023-12-20
- ↑ Nakagawa, Yoshinobu; Pooh, Kyonghon; Kobayashi, Toru; Kageji, Teruyoshi; Uyama, Shinichi; Matsumura, Akira; Kumada, Hiroaki (2003-03-01). "Clinical review of the japanese experience with boron neutron capture therapy and a proposed strategy using epithermal neutron beams". Journal of Neuro-Oncology. 62 (1): 87–99. doi:10.1007/BF02699936. ISSN 1573-7373. PMID 12749705. S2CID 24755949.
- ↑ Laramore, George E.; Spence, Alexander M. (1996). "Boron neutron capture therapy (BNCT) for high-grade gliomas of the brain: A cautionary note". International Journal of Radiation Oncology*Biology*Physics. 36 (1): 241–246. doi:10.1016/S0360-3016(96)00241-6. PMID 8823281.
- ↑ Vos, Maaike J.; Turowski, Bernd; Zanella, Friedhelm E.; Paquis, Philippe; Siefert, Axel; Hideghéty, Katalin; Haselsberger, Klaus; Grochulla, Frank; Postma, Tjeerd J.; Wittig, Andrea; Heimans, Jan J.; Slotman, Ben J.; Vandertop, W. Peter; Sauerwein, Wolfgang (2005). "Radiologic findings in patients treated with boron neutron capture therapy for glioblastoma multiforme within EORTC trial 11961". International Journal of Radiation Oncology*Biology*Physics. 61 (2): 392–399. doi:10.1016/j.ijrobp.2004.06.008. PMID 15667958.
- 1 2 3 Barth, Rolf F; H Vicente, MGraca; Harling, Otto K; Kiger, Ws; Riley, Kent J; Binns, Peter J; Wagner, Franz M; Suzuki, Minoru; Aihara, Teruhito; Kato, Itsuro; Kawabata, Shinji (2012). "Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer". Radiation Oncology. 7 (1): 146. doi:10.1186/1748-717X-7-146. ISSN 1748-717X. PMC 3583064. PMID 22929110.
- ↑ Snyder, H. R.; Reedy, Albert J.; Lennarz, Wm. J. (1958). "Synthesis of Aromatic Boronic Acids. Aldehydo Boronic Acids and a Boronic Acid Analog of Tyrosine 1". Journal of the American Chemical Society. 80 (4): 835–838. doi:10.1021/ja01537a021. ISSN 0002-7863.
- ↑ Mishima, Yutaka (1996), Mishima, Yutaka (ed.), "Selective Thermal Neutron Capture Therapy of Cancer Cells Using their Specific Metabolic Activities—Melanoma as Prototype", Cancer Neutron Capture Therapy, Boston, MA: Springer US, pp. 1–26, doi:10.1007/978-1-4757-9567-7_1, ISBN 978-1-4757-9567-7, retrieved 2023-12-21
- 1 2 Diaz, Aidnag Z. (2003). "Assessment of the results from the phase I/II boron neutron capture therapy trials at the Brookhaven National Laboratory from a clinician's point of view". Journal of Neuro-oncology. 62 (1–2): 101–109. doi:10.1007/BF02699937. ISSN 0167-594X. PMID 12749706. S2CID 31092140.
- 1 2 Luo, Ting; Huang, Wenzhi; Chu, Feiyi; Zhu, Tianyu; Feng, Bin; Huang, Shuai; Hou, Jing; Zhu, Liyong; Zhu, Shaihong; Zeng, Wenbin (2023-10-02). "The Dawn of a New Era: Tumor-Targeting Boron Agents for Neutron Capture Therapy". Molecular Pharmaceutics. 20 (10): 4942–4970. doi:10.1021/acs.molpharmaceut.3c00701. ISSN 1543-8384. PMID 37728998. S2CID 262086894.