6)%252B%252B%252B(BIGNOU01).png.webp)
In organic chemistry, an aza-crown ether is an aza analogue of a crown ether (cyclic polyether).[2][3] That is, it has a nitrogen atom (amine linkage, −NH− or >N−) in place of each oxygen atom (ether linkage, −O−) around the ring. While the parent crown ethers have the formulae (CH2CH2O)n, the parent aza-crown ethers have the formulae (CH2CH2NH)n, where n = 3, 4, 5, 6. Well-studied aza crowns include triazacyclononane (n = 3), cyclen (n = 4),[4] and hexaaza-18-crown-6 (n = 6).[5]
- Selected aza-crowns and their complexes
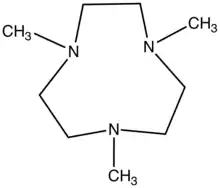 1,4,7-Trimethyl-1,4,7-triazacyclononane, a tridentate ligand used in coordination chemistry.
1,4,7-Trimethyl-1,4,7-triazacyclononane, a tridentate ligand used in coordination chemistry.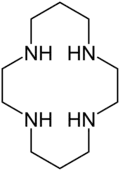
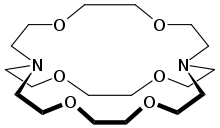 2.2.2-Cryptand is an aza-crown of the mixed ether-amine variety.
2.2.2-Cryptand is an aza-crown of the mixed ether-amine variety.
Synthesis
The synthesis of aza crown ethers are subject to the challenges associated with the preparation of macrocycles.[7] The 18-membered ring in (CH2CH2NH)6 can be synthesized by combining two triamine components.[5] By reaction with tosyl chloride, diethylene triamine is converted to a derivative with two secondary sulfonamides. This compound serves as a building block for macrocyclizations.
Variants
Many kinds of aza crown ethers exist.
- Variable length linkers
Aza crowns often feature trimethylene ((CH2)3) as well as ethylene ((CH2)2) linkages. One example is cyclam (1,4,8,11-tetraazacyclotetradecane).
- Tertiary amines
In many aza-crown ethers some or all of the amines are tertiary. One example is the tri(tertiary amine) (CH2CH2NCH3)3, known as trimethyltriazacyclononane. Cryptands, three-dimensional aza crowns, feature tertiary amines.
- Mixed ether-amine ligands
Another large class of macrocyclic ligands feature both ether and amines..[8] One example is the diaza-18-crown-6, [(CH2CH2O)2(CH2CH2NH)]2.[9]
- Lariate crowns
The presence of the amine allows the formation of Lariat crown ethers, which feature sidearms that augment complexation of cation.[10]
References
- ↑ Morooka, M.; Ohba, S.; Toriumi, K. (1992). "Electron-density distribution in crystals of 1,4,7,10,13,16-hexaazacyclooctadecanecobalt(III) trichloride, meso-[Co(hexaen)]Cl3 at 106 K". Acta Crystallographica Section B: Structural Science. 48 (4): 459–463. doi:10.1107/S0108768192002714.
- ↑ Bencini, Andrea; Bianchi, Antonio; Garcia-España, Enrique; Micheloni, Mauro; Ramirez, José Antonio (1999). "Proton coordination by polyamine compounds in aqueous solution". Coordination Chemistry Reviews. 188: 97–156. doi:10.1016/S0010-8545(98)00243-4.
- ↑ Reichenbach-Klinke, Roland; König, Burkhard (2002). "Metal complexes of azacrown ethers in molecular recognition and catalysis". Journal of the Chemical Society, Dalton Transactions (2): 121–130. doi:10.1039/b106367g.
- ↑ Reed, David P.; Weisman, Gary R. (2002). "1,4,7,10-Tetraazacyclododecane". Org. Synth. 78: 73. doi:10.15227/orgsyn.078.0073.
- 1 2 Atkins, T. J.; Richman, J. E.; Oettle, W. F. (1978). "1,4,7,10,13,16-Hexaazacyclooctadecane". Org. Synth. 58: 86. doi:10.15227/orgsyn.058.0086.
- ↑ "Plerixafor". Drugs in R&D. 8 (2): 113–119. 2007. doi:10.2165/00126839-200708020-00006. PMID 17324009.
- ↑ Krakowiak, Krzysztof E.; Bradshaw, Jerald S.; Zamecka-Krakowiak, Daria J. (1989). "Synthesis of aza-crown ethers". Chemical Reviews. 89 (4): 929–972. doi:10.1021/cr00094a008.
- ↑ Frensdorff, Hans K. (1971). "Stability constants of cyclic polyether complexes with univalent cations". Journal of the American Chemical Society. 93 (3): 600–606. doi:10.1021/ja00732a007.
- ↑ Gatto, Vincent J.; Miller, Steven R.; Gokel, George W. (1990). "4,13-Diaza-18-Crown-6". Organic Syntheses. 68: 227. doi:10.15227/orgsyn.068.0227.
- ↑ Gokel, G. W.; Barbour, L. J.; Ferdani, R.; Hu, J. (2002). "Lariat Ether Receptor Systems Show Experimental Evidence for Alkali Metal Cation Interactions". Acc. Chem. Res. 35 (10): 878–886. doi:10.1021/ar000093p. PMID 12379140.