 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
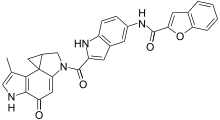
| C30H22N4O4 | |
| Molar mass | 502.530 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Adozelesin is an experimental antitumor drug of the duocarmycin class.[1] It binds to and alkylates DNA, resulting in a reduction of both cellular and simian virus 40 (SV40) DNA replication which ultimately reduces the rate of cancer growth.[2]
References
- ↑ Wang, Y; Beerman, TA; Kowalski, D (2001). "Antitumor drug adozelesin differentially affects active and silent origins of DNA replication in yeast checkpoint kinase mutants". Cancer Research. 61 (9): 3787–94. PMID 11325853.
- ↑ Liu, J.-S.; Kuo, S.-R.; McHugh, M. M.; Beerman, T. A.; Melendy, T. (14 January 2000). "Adozelesin Triggers DNA Damage Response Pathways and Arrests SV40 DNA Replication through Replication Protein A Inactivation". Journal of Biological Chemistry. 275 (2): 1391–1397. doi:10.1074/jbc.275.2.1391. PMID 10625690.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.