 | |
 | |
| Names | |
|---|---|
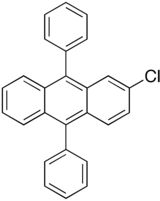
| Preferred IUPAC name
2-Chloro-9,10-diphenylanthracene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C26H17Cl | |
| Molar mass | 364.87 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2-Chloro-9,10-diphenylanthracene is a fluorescent dye used in glow sticks for a blue-green glow.[1] It is a chlorinated derivative of 9,10-diphenylanthracene.[2]
See also
References
- ↑ Jaworski, Jan S.; Leszczyński, Piotr; Filipek, Sławomir (1997-12-20). "Rates of the halide ion cleavage from halo-9,10-diphenylanthracene anion radicals in DMF". Journal of Electroanalytical Chemistry. 440 (1): 163–167. doi:10.1016/S0022-0728(97)80052-6. ISSN 1572-6657.
- ↑ Evans, John F.; Blount, Henry N. (February 1976). "Reactions of cation radicals of EE [electron capture] systems. III. Chlorination of 9,10-diphenylanthracene". The Journal of Organic Chemistry. 41 (3): 516–519. doi:10.1021/jo00865a022. ISSN 0022-3263.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.