 | |
| Names | |
|---|---|
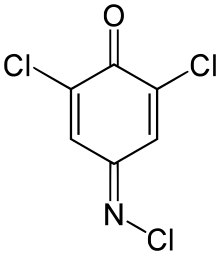
| Preferred IUPAC name
2,6-Dichloro-4-(chloroimino)cyclohexa-2,5-dien-1-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.671 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H2Cl3NO | |
| Molar mass | 210.44 g·mol−1 |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H242, H315, H319, H335 | |
| P210, P220, P234, P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P411, P420, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2,6-Dichloroquinone-4-chloroimide (Gibbs reagent) is an organic compound used as an colorimetric indicator to detect phenolic compounds.[1] Upon reaction with phenol itself, 2,6-dichlorophenolindophenol is formed,[2] a chemical that is used as a redox indicator.
References
- ↑ Arip, Mohamad Nasir Mat; Heng, Lee Yook; Ahmad, Musa; Aishah Hasbullah, Siti (2013). "Reaction of 2,6-dichloroquinone-4-chloroimide (Gibbs reagent) with permethrin – an optical sensor for rapid detection of permethrin in treated wood". Chem Cent J. 7: 122. doi:10.1186/1752-153X-7-122. PMC 3726330. PMID 23867006.
- ↑ Svobodová, D.; Křenek, P.; Fraenkl, M.; Gasparič, J. (1977). "Colour Reaction of Phenols with the Gibbs Reagent. The Reaction Mechanism and Decomposition and Stabilisation of the Reagent". Microchim. Acta. 67 (3–4): 251–264. doi:10.1007/BF01213035. S2CID 93587492.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.