 | |
| Clinical data | |
|---|---|
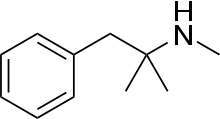
| Other names | methyl(2-methyl-1-phenylpropan-2-yl)amine |
| Routes of administration | IM/IV |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Rapidly demethylated in the body followed by hydroxylation. |
| Excretion | Via urine (as unchanged and metabolites); more rapid in acidic urine. |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.638 |
| Chemical and physical data | |
| Formula | C11H17N |
| Molar mass | 163.264 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Mephentermine (Wyamine, former brandname) is a cardiac stimulant.
It has been used as a treatment for low blood pressure.[1]
Mephentermine belongs to the class of adrenergic and dopaminergic cardiac stimulants excluding cardiac glycosides. Used in the treatment of heart failure.
Mechanism of action
Mephentermine appears to act by indirect stimulation of β-adrenergic receptors causing the release of norepinephrine from its storage sites. It has a positive inotropic effect on the myocardium. AV conduction and refractory period of AV node is shortened with an increase in ventricular conduction velocity. It dilates arteries and arterioles in the skeletal muscle and mesenteric vascular beds, leading to an increase in venous return.
Its onset is 5 to–15 minutes with intramuscular dosing, and immediate with intravenous dosing.
Its duration of action is four hours with intramuscular dosing and 30 minutes with intravenous dosing.
Indication and dosage
For maintenance of blood pressure in hypotensive states, the dose for adults is 30–45 mg as a single dose, repeated as necessary or followed by intravenous infusion of 0.1% mephentermine in 5% dextrose, with the rate and duration of administration depending on the patient's response.
For hypotension secondary to spinal anaesthesia in obstetric patients, the dose for adults is 15 mg as a single dose, repeated if needed. The maximum dose 30 mg.
Caution
Contraindications
Low blood pressure caused by phenothiazines, hypertension, and pheochromocytoma.
Special Precautions
Patients receiving monoamine oxidase inhibitors. For shock due to loss of blood or fluid, give fluid replacement therapy primarily, cardiovascular disease, hypertension, hyperthyroidism, chronic illnesses, lactation, pregnancy, skin dryness. headache.
Adverse Drug Reactions
The most common side effects are drowsiness, incoherence, hallucinations, convulsions, slow heart rate (reflex bradycardia). Fear, anxiety, restlessness, tremor, insomnia, confusion, irritability, and psychosis. Nausea, vomiting, reduced appetite, urinary retention, dyspnea, weakness, neck pain
Potentially fatal reactions are due to atrioventricular block, central nervous system stimulation, cerebral hemorrhage, pulmonary edema, and ventricular arrhythmias.
Drug Interactions
Mephentermine antagonizes effect of agents that lower blood pressure. Severe hypertension may occur with monoamine oxidase inhibitors and possibly tricyclic antidepressants. Additive vasoconstricting effects occur with ergot alkaloids, and oxytocin.
Potentially fatal drug interactions are the risk of abnormal heart rhythm in people undergoing anesthesia with cyclopropane and halothane.
Synthesis
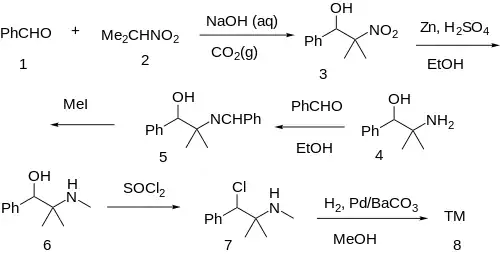
The Henry (Nitro-Aldol) reaction between Benzaldehyde [100-52-7] (1) and 2-Nitropropane [79-46-9] (2) gives 2-methyl-2-nitro-1-phenylpropan-1-ol [33687-74-0] (3). The nitro group is reduced with zinc in sulfuric acid giving 2-Phenyl-1,1-dimethylethanolamine [34405-42-0] (4). Imine formation by dehydration with benzaldehyde gives CID:12640087 (5). Alkylation with iodomethane led to CID:10058106 (6). Halogenation with thionyl chloride gave CID:129921490 (7). Lastly, a Rosenmund reduction completed the total synthesis of mephentermine (8).
According to JACS, phentermine was condensed with benzaldehyde to get the Schiff-base. This was then alkylated with methyl iodide to give mephentermine.[5]
See also
- Oxetacaine
- DAN-2817 [62099-12-1]
References
- ↑ Whittington RM (May 1963). "Mephentermine Sulphate as a Hypertensive Agent in General Practice". The Journal of the College of General Practitioners. 6 (2): 336–7. PMC 1878111. PMID 14000433.
- ↑ William F Bruce, Szabo Joseph Lester, Tubis Samuel, U.S. Patent 2,597,445 (1952 to Wyeth Corp).
- ↑ Data, John B.; Skibbe, Martin O.; Kerley, T. Lamar; Weaver, Lawrence C. (1966). "Synthesis of N-Substituted Phenethylamines and Corresponding Cyclohexyl Analogs". Journal of Pharmaceutical Sciences. 55 (1): 38–43. doi:10.1002/jps.2600550108.
- ↑ Harvey E Alburn, Donald E Clark, & Norman H Grant, U.S. Patent 3,300,510 (1967 to Wyeth LLC).
- ↑ Zenitz, Bernard L.; Macks, Elizabeth B.; Moore, Maurice L. (1948). "Preparation of α,α-Dimethyl- and N,α,α-Trimethyl-β-cyclohexylethylamine". Journal of the American Chemical Society. 70 (3): 955–957. doi:10.1021/ja01183a019.