 | |
| Clinical data | |
|---|---|
| Trade names | Vafseo |
| Other names | AKB-6548, PG-1016548 |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.248.991 |
| Chemical and physical data | |
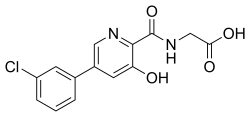
| Formula | C14H11ClN2O4 |
| Molar mass | 306.70 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Vadadustat, sold under the brand name Vafseo is a medication used for the treatment of symptomatic anemia associated with chronic kidney disease.[2]
The most common side effects include thromboembolic events (problems due to the formation of blood clots in the blood vessels), diarrhea, and hypertension (high blood pressure).[2]
Vadadustat was approved for medical use in the European Union in April 2023.[2]
Medical uses
Vadadustat is indicated for the treatment of symptomatic anemia associated with chronic kidney disease in adults on chronic maintenance dialysis.[2]
Society and culture
Legal status
On 23 February 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Vafseo, intended for the treatment of symptomatic anemia in adults with chronic kidney disease who are on chronic dialysis.[3] The applicant for this medicinal product is Akebia Europe Limited.[3] Vadadustat was approved for medical use in the European Union in April 2023.[2]
Research
Vadadustat is in phase III clinical trials for the treatment of anemia caused by chronic kidney disease.[4][5][6][7][8]
References
- 1 2 https://www.tga.gov.au/resources/auspmd/vafseo
- 1 2 3 4 5 6 "Vafseo EPAR". European Medicines Agency. 31 May 2023. Archived from the original on 3 June 2023. Retrieved 3 June 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2 "Vafseo: Pending EC decision". European Medicines Agency (EMA). 24 February 2023. Archived from the original on 25 February 2023. Retrieved 24 February 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ Pergola PE, Spinowitz BS, Hartman CS, Maroni BJ, Haase VH (November 2016). "Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease". Kidney International. 90 (5): 1115–1122. doi:10.1016/j.kint.2016.07.019. PMID 27650732.
- ↑ Gupta N, Wish JB (June 2017). "Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD". American Journal of Kidney Diseases. 69 (6): 815–826. doi:10.1053/j.ajkd.2016.12.011. PMID 28242135.
- ↑ Martin ER, Smith MT, Maroni BJ, Zuraw QC, deGoma EM (2017). "Clinical Trial of Vadadustat in Patients with Anemia Secondary to Stage 3 or 4 Chronic Kidney Disease". American Journal of Nephrology. 45 (5): 380–388. doi:10.1159/000464476. PMC 5452283. PMID 28343225.
- ↑ Eckardt KU, Agarwal R, Aswad A, Awad A, Block GA, Bacci MR, et al. (April 2021). "Safety and Efficacy of Vadadustat for Anemia in Patients Undergoing Dialysis". The New England Journal of Medicine. 384 (17): 1601–1612. doi:10.1056/NEJMoa2025956. PMID 33913638.
- ↑ Chertow GM, Pergola PE, Farag YM, Agarwal R, Arnold S, Bako G, et al. (April 2021). "Vadadustat in Patients with Anemia and Non-Dialysis-Dependent CKD". The New England Journal of Medicine. 384 (17): 1589–1600. doi:10.1056/NEJMoa2035938. PMID 33913637.