 | |
| Names | |
|---|---|
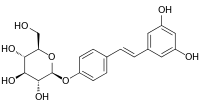
| IUPAC name
4-[(E)-2-(3,5-Dihydroxyphenyl)ethen-1-yl]phenyl β-D-glucopyranoside | |
| Systematic IUPAC name
(2S,3R,4S,5S,6R)-2-{4-[(E)-2-(3,5-Dihydroxyphenyl)ethen-1-yl]phenoxy}-6-(hydroxymethyl)oxane-3,4,5-triol | |
| Other names
trans-Resveratrol-4'-O-beta-D-glucopyranoside (E)-Resveratroloside; 3,5,4'-Trihydroxystilbene-4'-glucoside | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H22O8 | |
| Molar mass | 390.388 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Resveratroloside is a stilbenoid glucoside. It can be found in Paeonia lactiflora.[1]
References
- ↑ Kim, H. J.; Chang, E. J.; Cho, S. H.; Chung, S. K.; Park, H. D.; Choi, S. W. (2002). "Antioxidative activity of resveratrol and its derivatives isolated from seeds of Paeonia lactiflora". Bioscience, Biotechnology, and Biochemistry. 66 (9): 1990–1993. doi:10.1271/bbb.66.1990. PMID 12400706. S2CID 24367582.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.