 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌsʌksɪnɪlˈkoʊliːn/ |
| Trade names | Quelicin, Anectine, others |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Metabolism | By pseudocholinesterase, to succinylmonocholine and choline |
| Onset of action | 30–60 sec (IV), 2–3 min (IM) |
| Duration of action | < 10 min (IV), 10–30 min (IM) |
| Excretion | Kidney (10%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
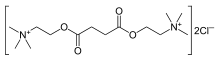
| Formula | C14H30Cl2N2O4 |
| Molar mass | 361.30 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Suxamethonium chloride, [Scoline, Sucostrin] also known as suxamethonium or succinylcholine, or simply sux by medical abbreviation,[4] is a medication used to cause short-term paralysis as part of general anesthesia.[5] This is done to help with tracheal intubation or electroconvulsive therapy.[5] It is administered by injection, either into a vein or into a muscle.[6] When used in a vein, onset of action is generally within one minute and effects last for up to 10 minutes.[6]
Common side effects include low blood pressure, increased saliva production, muscle pain, and rash.[6] Serious side effects include malignant hyperthermia, hyperkalemia and allergic reactions.[7][8] It is not recommended in people who are at risk of high blood potassium or a history of myopathy.[5] Use during pregnancy appears to be safe for the baby.[9]
Suxamethonium is in the neuromuscular blocker family of medications and is of the depolarizing type.[6] It works by blocking the action of acetylcholine on skeletal muscles.[6]
Suxamethonium was described as early as 1906 and came into medical use in 1951.[4] It is on the World Health Organization's List of Essential Medicines.[10] Suxamethonium is available as a generic medication.[6]
Medical uses
Succinylcholine chloride injection is indicated, in addition to general anesthesia, to facilitate tracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation.[8]
Its medical uses are limited to short-term muscle relaxation in anesthesia and intensive care, usually for facilitation of endotracheal intubation. It is popular in emergency medicine due to its rapid onset and brief duration of action. The former is a major point of consideration in the context of trauma care, where endotracheal intubation may need to be completed very quickly. The latter means that, should attempts at endotracheal intubation fail and the person cannot be ventilated, there is a prospect for neuromuscular recovery and the onset of spontaneous breathing before low blood oxygen levels occurs. It may be better than rocuronium in people without contraindications due to its faster onset of action and shorter duration of action.[11]
Suxamethonium is also commonly used as the sole muscle relaxant during electroconvulsive therapy, favoured for its short duration of action.[12]
Suxamethonium is quickly degraded by plasma butyrylcholinesterase and the duration of effect is usually in the range of a few minutes. When plasma levels of butyrylcholinesterase are greatly diminished or an atypical form is present (an otherwise harmless inherited disorder), paralysis may last much longer, as is the case in liver failure or in neonates.[13]
The vials are usually stored at a temperature between 2–8 °C, but issues have been reported with lower storage temperatures.[14] The multi-dose vials are stable for up to 14 days at room temperature without significant loss of potency.[3] Unless otherwise indicated in the prescribing information, room temperature for storage of medications is 15–25 °C (59–77 °F).[15]
Side effects
Side effects include malignant hyperthermia, muscle pains, acute rhabdomyolysis with high blood levels of potassium,[13] transient ocular hypertension, constipation[16] and changes in cardiac rhythm, including slow heart rate, and cardiac arrest. In people with neuromuscular disease or burns, an injection of suxamethonium can lead to a large release of potassium from skeletal muscles, potentially resulting in cardiac arrest. Conditions having susceptibility to suxamethonium-induced high blood potassium are burns, closed head injury, acidosis, Guillain–Barré syndrome, cerebral stroke, drowning, severe intra-abdominal sepsis, massive trauma, myopathy, and tetanus.
Suxamethonium does not produce unconsciousness or anesthesia, and its effects may cause considerable psychological distress while simultaneously making it impossible for a patient to communicate. Therefore, administration of the drug to a conscious patient is contraindicated.
Hyperkalemia
The side effect of high blood potassium may occur because the acetylcholine receptor is propped open, allowing continued flow of potassium ions into the extracellular fluid. A typical increase of potassium ion serum concentration on administration of suxamethonium is 0.5 mmol per liter.The increase is transient in otherwise healthy patients. The normal range of potassium is 3.5 to 5 mEq per liter. High blood potassium does not generally result in adverse effects below a concentration of 6.5 to 7 mEq per liter. Therefore, the increase in serum potassium level is usually not catastrophic in otherwise healthy patients. Severely high blood levels of potassium can cause changes in cardiac electrophysiology, which, if severe, can result in arrhythmias and even cardiac arrest.[17][18]
Malignant hyperthermia
Malignant hyperthermia (MH) from suxamethonium administration can result in a drastic and uncontrolled increase in skeletal muscle oxidative metabolism. This overwhelms the body's capacity to supply oxygen, remove carbon dioxide, and regulate body temperature, eventually leading to circulatory collapse and death if not treated quickly.
Susceptibility to malignant hyperthermia is often inherited as an autosomal dominant disorder, for which there are at least six genetic loci of interest, the most prominent being the ryanodine receptor gene (RYR1). MH susceptibility is phenotype and genetically related to central core disease (CCD), an autosomal dominant disorder characterized both by MH symptoms and by myopathy. MH is usually unmasked by anesthesia, or when a family member develops the symptoms. There is no simple, straightforward test to diagnose the condition. When MH develops during a procedure, treatment with dantrolene sodium is usually initiated; dantrolene and the avoidance of suxamethonium administration in susceptible people have markedly reduced the mortality from this condition.
Apnea
The normal short duration of action of suxamethonium is due to the rapid metabolism of the drug by non-specific plasma cholinesterases. However plasma cholinesterase activity is reduced in some people due to either genetic variation or acquired conditions, which results in a prolonged duration of neuromuscular block. Genetically, ninety six percent of the population have a normal (Eu:Eu) genotype and block duration; however, some people have atypical genes (Ea, Es, Ef) which can be found in varying combinations with the Eu gene, or other atypical genes (see Pseudocholinesterase deficiency). Such genes will result in a longer duration of action of the drug, ranging from 20 minutes up to several hours. Acquired factors that affect plasma cholinesterase activity include pregnancy, liver disease, kidney failure, heart failure, thyrotoxicosis, and cancer, as well as a number of other drugs.[19]
If unrecognized by a clinician it could lead to awareness if anesthesia is discontinued whilst still paralyzed or hypoxemia (and potentially fatal consequences) if artificial ventilation is not maintained. Normal treatment is to maintain sedation and ventilate the patient on an intensive care unit until muscle function has returned. Blood testing for cholinesterase function can be performed.
Mivacurium, a non-depolarizing neuromuscular blocking drug, is also metabolized via the same route with a similar clinical effect in patients deficient in plasma cholinesterase activity.
Deliberate induction of conscious apnea using this drug led to its use as a form of aversion therapy in the 1960s and 1970s in some prison and institutional settings.[20][21][22] This use was discontinued after negative publicity concerning the terrifying effects on subjects of this treatment and ethical questions about the punitive use of painful aversion.
Mechanism of action
There are two phases to the blocking effect of suxamethonium.
Phase 1 block
Phase 1 blocking has the principal paralytic effect. Binding of suxamethonium to the nicotinic acetylcholine receptor results in opening of the receptor's monovalent cation channel; a disorganized depolarization of the motor end-plate occurs and calcium is released from the sarcoplasmic reticulum.[23]
In normal skeletal muscle, acetylcholine dissociates from the receptor following depolarization and is rapidly hydrolyzed by acetylcholinesterase. The muscle cell is then ready for the next signal.[23]
Suxamethonium has a longer duration of effect than acetylcholine, and is not hydrolyzed by acetylcholinesterase. By maintaining the membrane potential above threshold, it does not allow the muscle cell to repolarize. When acetylcholine binds to an already depolarized receptor, it cannot cause further depolarization. [23]
Calcium is removed from the muscle cell cytoplasm independent of repolarization (depolarization signaling and muscle contraction are independent processes). As the calcium is taken up by the sarcoplasmic reticulum, the muscle relaxes. This explains muscle flaccidity rather than tetany following fasciculations.
The results are membrane depolarization and transient fasciculations, followed by flaccid paralysis.
Phase 2 block
While this phase is not abnormal and is a part of its mechanism of action, it is undesirable during surgery, due to the inability to depolarize the cell again.[23] Often, patients must be on a ventilator for hours if Phase 2 block occurs. It generally occurs when suxamethonium is administered multiple times, or during an infusion occurring over too much time, but can also occur during an initial bolus if the plasma cholinesterase is abnormal[23] Desensitization may occur at the nerve terminal causing the myocyte becomes less sensitive to acetylcholine, resulting in the membrane repolarizing and being unable be depolarized again for a period of time.[23]
Chemistry
Suxamethonium is an odorless, white crystalline substance. Aqueous solutions have a pH of about 4. The dihydrate melts at 160 °C, whereas the anhydrous melts at 190 °C. It is highly soluble in water (1 gram in about 1 mL), soluble in ethyl alcohol (1 gram in about 350 mL), slightly soluble in chloroform, and practically insoluble in ether. Suxamethonium is a hygroscopic compound.[24] The compound consists of two acetylcholine molecules that are linked by their acetyl groups. It can also be viewed as a central moiety of succinic acid with two choline moieties, one on each end.
History
Suxamethonium was first discovered in 1906 by Reid Hunt and René de M. Taveau. When studying the drug, animals were given curare and thus they missed the neuromuscular blocking properties of suxamethonium. Instead in 1949 an Italian group led by Daniel Bovet was first to describe succinylcholine induced paralysis. The clinical introduction of suxamethonium was described in 1951 by several groups. Papers published by Stephen Thesleff and Otto von Dardel in Sweden are important but also to be mentioned is work by Bruck, Mayrhofer and Hassfurther in Austria, Scurr and Bourne in UK, and Foldes in America.[25]
Abuse
Dubai authorities deem that the murder of Hamas operative Mahmoud al-Mabhouh was carried out on their soil by Mossad agents with the use of suxamethonium chloride injection. Entering Dubai under false passports in 2010, the Mossad agents found al-Mabhouh at a hotel, immobilized him with the drug, electrocuted him, then suffocated him by pillow in an assassination. A high concentration of suxamethonium chloride was found in al-Mabhouh's body post-mortem. The incident triggered significant diplomatic crises in the Middle East, Europe, and Australia.[26][27]
It was used by serial killer Efren Saldivar (1988–1998), and in the murder of Kathy Augustine (2006).
Brand names
It is available in German-speaking countries under the trade name Lysthenon among others.[28]
Other animals
It is sometimes used in combination with pain medications and sedatives for euthanasia and immobilization of horses.
References
- ↑ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ↑ "Anectine- succinylcholine chloride injection, solution". DailyMed. U.S. National Library of Medicine. 17 September 2018. Retrieved 23 November 2020.
- 1 2 "Quelicin- succinylcholine chloride injection, solution". DailyMed. U.S. National Library of Medicine. 15 February 2019. Retrieved 23 November 2020.
- 1 2 Lee C, Katz RL (March 2009). "Clinical implications of new neuromuscular concepts and agents: so long, neostigmine! So long, sux!". Journal of Critical Care. 24 (1): 43–49. doi:10.1016/j.jcrc.2008.08.009. PMID 19272538.
- 1 2 3 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 426–8. hdl:10665/44053. ISBN 9789241547659.
- 1 2 3 4 5 6 "Succinylcholine Chloride". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ↑ "Anectine Injection - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. 12 January 2016. Archived from the original on 20 December 2016. Retrieved 16 December 2016.
- 1 2 "Coronavirus (COVID-19) Update: December 22, 2020". U.S. Food and Drug Administration (Press release). 22 December 2020. Retrieved 23 December 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Prescribing medicines in pregnancy database". Therapeutic Goods Administration (TGA). 16 December 2016. Archived from the original on 20 December 2016. Retrieved 16 December 2016.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Tran DT, Newton EK, Mount VA, Lee JS, Wells GA, Perry JJ (October 2015). "Rocuronium versus succinylcholine for rapid sequence induction intubation". The Cochrane Database of Systematic Reviews. 2015 (10): CD002788. doi:10.1002/14651858.CD002788.pub3. PMC 7104695. PMID 26512948.
- ↑ Lee C (March 2009). "Goodbye suxamethonium!". Anaesthesia. 64 Suppl 1: 73–81. doi:10.1111/j.1365-2044.2008.05873.x. PMID 19222434.
- 1 2 Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G (25 March 2011). Rang and Dale's Pharmacology (7th ed.). Elsevier Science Health Science Division. ISBN 978-0-7020-3471-8. Archived from the original on 10 September 2017.
- ↑ Dewachter P, Mouton-Faivre C (February 2016). "Frozen succinylcholine: the danger of being overzealous with its cold storage". Br J Anaesth. 116 (2): 299–300. doi:10.1093/bja/aev465. PMID 26787804.
- ↑ "Guidelines for the Storage of Essential Medicines and Other Health Commodities: 3. Maintaining the Quality of Your Products: Controlling temperature". apps.who.int. World Health Organization. Archived from the original on May 25, 2011. Retrieved 2020-03-10.
- ↑ DiPiro JT, Talbert RL, Yee GC (2005). Matzke Pharmacotherapy: A Pathophysiologicḣ Approach (6th ed.). McGraw-Hill. p. 685.
- ↑ Hager HH, Burns B (2022). "Succinylcholine Chloride". StatPearls. PMID 29763160.
- ↑ Martyn JA, Richtsfeld M (January 2006). "Succinylcholine-induced hyperkalemia in acquired pathologic states: etiologic factors and molecular mechanisms". Anesthesiology. 104 (1): 158–169. doi:10.1097/00000542-200601000-00022. PMID 16394702. S2CID 4556150.
- ↑ Peck TE, Hill SA, Williams M (2003). Pharmacology for Anaesthesia and Intensive Care (2nd ed.). London, UK: Greenwich Medical Media Limited. ISBN 1-84110-166-4.
- ↑ Reimringer MJ, Morgan SW, Bramwell PF (1970). "Succinylcholine as a modifier of acting-out behavior". Clinical Medicine. 77 (7): 28.
- ↑ von Hoffman N (5 April 1972). "A Bit of 'Clockwork Orange,' California-Style". Washington Post.
- ↑ Sansweet RJ (1975). The Punishment Cure. New York: Mason/Charter. ISBN 0-88405-118-8.
- 1 2 3 4 5 6 Appiah-Ankam J, Hunter JM (February 2004). "Pharmacology of neuromuscular blocking drugs". Continuing Education in Anaesthesia Critical Care & Pain. 4 (1): 2–7. doi:10.1093/bjaceaccp/mkh002.
- ↑ Gennaro A (2000). The Science and Practice of Pharmacy (20th ed.). Remington: Lippincott Williams & Wilkins. p. 1336.
- ↑ Dorkins HR (April 1982). "Suxamethonium-the development of a modern drug from 1906 to the present day". Medical History. 26 (2): 145–168. doi:10.1017/S0025727300041132. PMC 1139149. PMID 7047939.
- ↑ "Dubai Hit: Police Say They Know How Mahmoud al-Mabhouh Was Killed". ABC News.
- ↑ "Succinylcholine, A Perfect Poison, Makes Appearance in the Dubai Killing". Medgadget. 8 March 2010.
- ↑ Compendium.ch: LYSTHENON 2% Inj Lös 100 mg/5ml (in German)
External links
- "Suxamethonium Chloride". Drug Information Portal. U.S. National Library of Medicine.