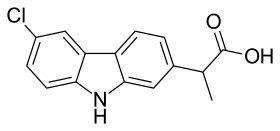
 Carprofen molecule | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | FDA Professional Drug Information |
| Routes of administration | Oral, injection |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | High (99%) |
| Elimination half-life | Approximately 8 h (range 4.5–9.8 h) in dogs |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.053.357 |
| Chemical and physical data | |
| Formula | C15H12ClNO2 |
| Molar mass | 273.72 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Carprofen is a nonsteroidal anti-inflammatory drug (NSAID) of the carbazole and propionic acid class that was previously for use in humans and animals but is now only available to veterinarians for prescribing as a supportive treatment for various conditions in animals.[1] Carprofen reduces inflammation by inhibition of COX-1 and COX-2; its specificity for COX-2 varies from species to species.[1] Marketed under many brand names worldwide,[2] carprofen is used as a treatment for inflammation and pain, including joint pain and postoperative pain.[1]
Human use
Carprofen was used in humans for almost ten years, starting in 1988, for the same conditions as in dogs; namely, joint pain and inflammation. Side effects tended to be mild, usually consisting of nausea or gastrointestinal pain and diarrhoea. It was available by prescription in 150 mg to 600 mg doses.[3] Dosages over 250 mg were reserved for pain caused by severe trauma, such as postoperative inflammation; 150 mg doses were commonly used to relieve arthritis pain, while 200 mg doses were commonly prescribed for severe arthritis or inflammatory pain. The drug was taken orally.
Pfizer voluntarily removed the medication from the market for human use on commercial grounds.[3]
Veterinary medicine
Canine use
Carprofen is one of eleven nonsteroidal anti-inflammatory drugs approved for use in dogs.[4] It aids in the relief of inflammation, pain, and fever. Carprofen can be administered in pill, chewable tablet, or injection form.[5]
Carprofen can be used for long-term pain management of such conditions as osteoarthritis, which is common in canine patients, or after surgical procedures for relief of acute pain and inflammation.[5][6]
In patients suffering from pain, carprofen has been shown to improve energy, activity level, comfort, and general well-being.[6]
Adverse effects
Most dogs respond well to carprofen use, but like all NSAIDs, it can cause gastrointestinal, liver, and kidney problems.[7]
In 1999, the Food and Drug Administration (FDA) received more than six thousand anecdotal reports of sudden animal death after usage of Pfizer's Rimadyl brand of carprofen. In response, the FDA requested that Pfizer advise consumers in their advertising that death is a possible side effect;[8] Pfizer refused and pulled their advertising, later including death as a possible side effect on the drug label.[9]
Adverse effects can include:
- Loss of appetite
- Vomiting
- Diarrhea
- Increase in thirst
- Increase in urination
- Fatigue and/or lethargy (drowsiness)
- Loss of coordination
- Seizures
- Liver dysfunction: jaundice (yellowing of eyes)
- Blood or dark tarry material in urine or stools
- Lethargy
- Staggering, stumbling, weakness or partial paralysis, full paralysis[10]
- Change in skin (redness, scabs, or scratching)
- Change in behavior (such as decreased or increased activity level, seizure or aggression)[11]
- In rare situations, death has been associated with some of the adverse reactions listed above.[12]
Effects of overdose include gastritis and ulcer formation.[13]
In healthy dogs given carprofen, no perioperative adverse effects on the cardiovascular system have been reported at recommended dosages.[14][15] Perioperative administration of carprofen to cats did not affect postoperative respiratory rate nor heart rate.[16]
Carprofen should not be administered concurrently with steroids, as this can cause ulcers in the stomach. Dogs should be taken off carprofen for three full days before ingesting a steroid (such as prednisolone). Carprofen should not be given at the same time with other types of medications, such as other NSAIDs (aspirin, etodolac, deracoxib, meloxicam, tepoxalin), or steroids such as dexamethasone, triamcinolone, cortisone, or prednisone.
Carprofen must be used with caution within the supervision of a veterinarian in dogs with liver or kidney disease, dehydration, bleeding deficits, or other health problems. It is not recommended for use in dogs with bleeding disorders (such as Von Willebrand's disease), as safety has not been established in dogs with these disorders.[17] It has not been established whether carprofen can be safely used in pregnant dogs, dogs used for breeding purposes, or in lactating dogs.
Several laboratory studies and clinical trials have been conducted to establish the safety of using carprofen. Clinical studies were conducted in nearly 300 dogs of different breeds. The dogs were treated with Rimadyl at the recommended dose for two weeks. According to these studies, the drug was clinically well tolerated, and the treated dogs did not have a greater incidence of adverse reactions when compared to the control group.[18]
A number of factors may contribute to the high incidence of adverse reports received for carprofen by the Center for Veterinary Medicine in the late 1990s. These include:
- The type of drug;
- Wide use;
- Duration of use. Long-term use can result in a higher risk for adverse reactions. It is recommended that blood tests for liver and kidney function are performed both prior to starting and regularly while on NSAIDs to monitor the patient’s tolerance;[19]
- Senior dog use. Older dogs are generally more prone to side effects caused by carprofen.
Equine use
Carprofen may be administered intravenously to horses.[20] A single dose has been shown to reduce prostaglandin E2 production and inflammatory exudate for up to 15 hours,[21] although there was less effect on eicosanoid production when compared to the effects produced by NSAIDs such as phenylbutazone or flunixin.[22] Prostaglandin E2 and inflammatory exudate are also reduced and leukotriene B4 is inhibited. Carprofen can also be given orally, but intramuscular use may produce muscle damage.[23]
Brands and dosage forms for veterinary use
It is marketed under many brand names including: Acticarp, Artriofin, Austiofen, Bomazeal, Canidryl, Carporal, Carprieve, Carprocow, Carprodolor, Carprodyl, Carprofelican, Carprofen, Carprofène, Carprofeno, Carprofenum, Carprogesic, Carprosol, Carprotab, Carprox, Comforion, Dolagis, Dolocarp, Dolox, Eurofen, Kelaprofen, Librevia, Norocarp, Norodyl, Novocox, Ostifen, Prolet, Quellin, Reproval, Rimadyl, Rimifin, Rofeniflex, Rovera, Rycarfa, Scanodyl, Tergive, Vetprofen, and Xelcor.[2]
Veterinary dosage forms include 25 mg, 75 mg, and 100 mg tablets, and 50 mg per mL injectable form.[1][24]
References
- 1 2 3 4 5 "Rimadyl (carprofen) prescribing information leaflet" (PDF). www2.ZoetisUS.com. Zoetis Inc. Retrieved 5 February 2022.
- 1 2 "International brand names for Carprofen". www.Drugs.com. Drugs.com. Retrieved 4 October 2017.
- 1 2 "Committee for Veterinary Medicinal Products: Carprofen" (PDF). www.EMA.Europa.eu. European Agency for the Evaluation of Medicinal Products. 2009.
- ↑ "Animal Drugs @ FDA - NSAID labels". AnimalDrugsAtFDA.FDA.gov. US FDA. Retrieved 16 November 2021.
- 1 2 Burke A (10 November 2016). "Rimadyl for Dogs – uses, side effects & alternatives". www.AKC.org. American Kennel Club. Retrieved 16 November 2021.
- 1 2 "Rimadyl". www.ZoetisPetCare.com. Retrieved 16 November 2021.
- ↑ Reimer ME, Johnston SA, Leib MS, Duncan RB, Reimer DC, Marini M, Gimbert K (September 1999). "The gastroduodenal effects of buffered aspirin, carprofen, and etodolac in healthy dogs". Journal of Veterinary Internal Medicine. 13 (5): 472–477. doi:10.1111/j.1939-1676.1999.tb01465.x. PMID 10499732.
- ↑ "Update on Rimadyl". www.FDA.gov. FDA's Center for Veterinary Medicine. 1 December 1999. Archived from the original on 8 September 2014. Retrieved 16 December 2019.
- ↑ Luna SP, Basílio AC, Steagall PV, Machado LP, Moutinho FQ, Takahira RK, Brandão CV (March 2007). "Evaluation of adverse effects of long-term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs". American Journal of Veterinary Research. 68 (3): 258–264. doi:10.2460/ajvr.68.3.258. PMID 17331014.
- ↑ "A review of signs of a potentially life-threatening reaction to Rimadyl". www.srdogs.com. Retrieved 20 May 2010.
- ↑ "Dog owner information about Rimadyl (carprofen)". www.Rimadyl.com. Pfizer. Archived from the original on 15 July 2011. Retrieved 20 May 2010.
- ↑ "Carprofen For Dogs: Dosage, Side Effects, And Alternatives". Relievet. Retrieved 2022-07-21.
- ↑ "Generic dog Rimadyl online". RimadylOnline.com. Archived from the original on 15 July 2011. Retrieved 15 July 2011.
- ↑ Boström IM, Nyman GC, Lord PE, Häggström J, Jones BE, Bohlin HP (May 2002). "Effects of carprofen on renal function and results of serum biochemical and hematologic analyses in anesthetized dogs that had low blood pressure during anesthesia". American Journal of Veterinary Research. 63 (5): 712–721. doi:10.2460/ajvr.2002.63.712. PMID 12013473.
- ↑ Frendin JH, Boström IM, Kampa N, Eksell P, Häggström JU, Nyman GC (December 2006). "Effects of carprofen on renal function during medetomidine-propofol-isoflurane anesthesia in dogs". American Journal of Veterinary Research. 67 (12): 1967–1973. doi:10.2460/ajvr.67.12.1967. PMID 17144795.
- ↑ Höglund OV, Dyall B, Gräsman V, Edner A, Olsson U, Höglund K (October 2018). "Effect of non-steroidal anti-inflammatory drugs on postoperative respiratory and heart rate in cats subjected to ovariohysterectomy". Journal of Feline Medicine and Surgery. 20 (10): 980–984. doi:10.1177/1098612X17742290. PMID 29165006. S2CID 30649716.
- ↑ "Rimadyl (Carprofen)". www.VetDepot.com. Vet Depot. Archived from the original on 23 May 2010. Retrieved 20 May 2010.
- ↑ "Rimadyl [package insert]" (PDF). www.Rimadyl.com. New York: Pfizer Animal Health. 2007. Retrieved 13 August 2014.
- ↑ "Get the facts about pain relievers for pets". www.FDA.gov. Center for Veterinary Medicine. 3 November 2020.
- ↑ McIlwraith CW, Frisbie DD, Kawcak CE (2001). "Nonsteroidal Anti-Inflammatory Drugs". Proc. AAEP (47): 182–187.
- ↑ Lees P, McKellar Q, May SA, Ludwig B (May 1994). "Pharmacodynamics and pharmacokinetics of carprofen in the horse". Equine Veterinary Journal. 26 (3): 203–208. doi:10.1111/j.2042-3306.1994.tb04370.x. PMID 8542839.
- ↑ Lees P, Ewins CP, Taylor JB, Sedgwick AD (1987). "Serum thromboxane in the horse and its inhibition by aspirin, phenylbutazone and flunixin". The British Veterinary Journal. 143 (5): 462–476. doi:10.1016/0007-1935(87)90024-8. PMID 3119142.
- ↑ McKellar QA, Bogan JA, von Fellenberg RL, Ludwig B, Cawley GD (July 1991). "Pharmacokinetic, biochemical and tolerance studies on carprofen in the horse". Equine Veterinary Journal. 23 (4): 280–284. doi:10.1111/j.2042-3306.1991.tb03718.x. PMID 1915228.
- ↑ "Carprofen (Veterinary – Systemic)" (PDF). The United States Pharmacopeial Convention. 2007.
External links
![]() Media related to Carprofen at Wikimedia Commons
Media related to Carprofen at Wikimedia Commons
