 | |
| Names | |
|---|---|
| IUPAC name
rhenium heptafluoride, heptafluoridorhenium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| ReF7 | |
| Molar mass | 319.196 g/mol |
| Appearance | Bright yellow crystalline solid |
| Density | 4.3 g/cm3 |
| Melting point | 48.3 °C (118.9 °F; 321.4 K) |
| Boiling point | 73.72 °C (164.70 °F; 346.87 K) |
| Reacts | |
| Vapor pressure | 13.41 kPa[1] |
| Structure | |
| triclinic, aP16 | |
| P1 (No. 2) | |
| Thermochemistry | |
Enthalpy of fusion (ΔfH⦵fus) |
7.53 kJ/mol[1] |
Enthalpy of vaporization (ΔfHvap) |
30.77 kJ/mol[1] |
| Related compounds | |
Related compounds |
Osmium heptafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
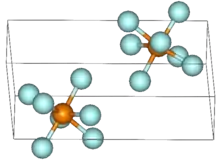
Rhenium heptafluoride is the compound with the formula ReF7. It is a yellow low melting solid and is the only thermally stable metal heptafluoride.[2] It has a distorted pentagonal bipyramidal structure similar to IF7, which was confirmed by neutron diffraction at 1.5 K.[3] The structure is non-rigid, as evidenced by electron diffraction studies.[4]
Production, reactions and properties
Rhenium heptafluoride can be prepared from the elements at 400 °C:[5]
- 2 Re + 7 F2 → 2 ReF7
It also can be produced by the explosion of rhenium metal under sulfur hexafluoride. [6]
It hydrolyzes under a base to form perrhenic acid and hydrogen fluoride:[1]
- ReF7 + 4H2O → HReO4 + 7HF
With fluoride donors such as CsF, the ReF−
8 anion is formed, which has a square antiprismatic structure.[7] With antimony pentafluoride, SbF5, a fluoride acceptor, the ReF+
6 cation is formed.[5]
References
- 1 2 3 4 J.G.Malm; H.Selig (1961). "The vapour-pressures and other properties of ReF6 and ReF7". Journal of Inorganic and Nuclear Chemistry. 20 (3): 189–197. doi:10.1016/0022-1902(61)80267-4.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Vogt T.; Fitch A. N.; Cockcroft J. K. (1994). "Crystal and Molecular Structures of Rhenium Heptafluoride". Science. 263 (5151): 1265–7. Bibcode:1994Sci...263.1265V. doi:10.1126/science.263.5151.1265. PMID 17817431. S2CID 20013073.
- ↑ Jacob, E. Jean; Bartell, L.S.J. (1970). "Electron Diffraction Study of Rhenium Fluorides. II. Structure, Pseudorotation, and Anharmonic Coupling of Modes in ReF7" (PDF). The Journal of Chemical Physics. 53 (6): 2235. Bibcode:1970JChPh..53.2235J. doi:10.1063/1.1674318. hdl:2027.42/70852.
- 1 2 A. F. Holleman; Wiberg, Egon; Wiberg, Nils (2001). Inorganic Chemistry. Boston: Academic Press. ISBN 0-12-352651-5.
- ↑ Richard L. Johnson; Bernard Siegel (1969). "On the synthesis of ReF7 and the existence of ReF2 and ReF3". Journal of Inorganic and Nuclear Chemistry. 31 (8): 2391–2396. doi:10.1016/0022-1902(69)80569-5.
- ↑ Hwang, I; Seppelt, K. (2000). "The structures of ReF−
8 and UF2−
8". Journal of Fluorine Chemistry. 102 (1–2): 69–72. doi:10.1016/S0022-1139(99)00248-1.