 | |
| Clinical data | |
|---|---|
| Trade names | Methosarb, Riedemil |
| Other names | 7β,17α-Dimethyltestosterone; NSC-88536; U-22550 |
| Routes of administration | By mouth |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
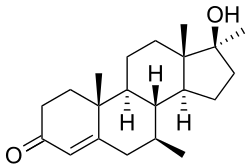
| Formula | C21H32O2 |
| Molar mass | 316.485 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Calusterone (INN, USAN) (brand names Methosarb, Riedemil; former developmental code names NSC-88536, U-22550), also known as 7β,17α-dimethyltestosterone, is an orally active anabolic-androgenic steroid (AAS) that is used as an antineoplastic agent.[1][2] It is a 17α-alkylated AAS similar in structure to bolasterone (which is its 7α-isomer).[1]
| Route | Medication | Form | Dosage | |
|---|---|---|---|---|
| Oral | Methyltestosterone | Tablet | 30–200 mg/day | |
| Fluoxymesterone | Tablet | 10–40 mg 3x/day | ||
| Calusterone | Tablet | 40–80 mg 4x/day | ||
| Normethandrone | Tablet | 40 mg/day | ||
| Buccal | Methyltestosterone | Tablet | 25–100 mg/day | |
| Injection (IM or SC) | Testosterone propionate | Oil solution | 50–100 mg 3x/week | |
| Testosterone enanthate | Oil solution | 200–400 mg 1x/2–4 weeks | ||
| Testosterone cypionate | Oil solution | 200–400 mg 1x/2–4 weeks | ||
| Mixed testosterone esters | Oil solution | 250 mg 1x/week | ||
| Methandriol | Aqueous suspension | 100 mg 3x/week | ||
| Androstanolone (DHT) | Aqueous suspension | 300 mg 3x/week | ||
| Drostanolone propionate | Oil solution | 100 mg 1–3x/week | ||
| Metenolone enanthate | Oil solution | 400 mg 3x/week | ||
| Nandrolone decanoate | Oil solution | 50–100 mg 1x/1–3 weeks | ||
| Nandrolone phenylpropionate | Oil solution | 50–100 mg/week | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | ||||
Calusterone is on the World Anti-Doping Agency's list of prohibited substances,[3] and is therefore banned from use in most major sports.
References
- 1 2 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 646–. ISBN 978-1-4757-2085-3.
- ↑ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 52–. ISBN 978-94-011-4439-1.
- ↑ "The World Anti-Doping Code: The 2020 Prohibited List" (PDF). World Anti-Doping Agency. Retrieved 2019-12-28.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.