 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| IrS2 | |
| Molar mass | 256.349 |
| Density | 9300 kg m–3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
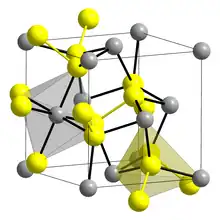
Iridium disulfide is the binary inorganic compound with the formula IrS2. Prepared by the direct reaction of the elements, the compound adopts the pyrite crystal structure at high pressure.[1] At normal atmospheric pressures, an orthorhombic polymorph is observed.[2]. The high- and low-pressure forms both feature octahedral Ir centers, but the S–S distances are pressure dependent.[3] Although not practical, IrS2 is a highly active catalyst for hydrodesulfurization.[4]
References
- ↑ Munson, Ronald A. (February 1968). "The synthesis of iridium disulfide and nickel diarsenide having the pyrite structure" (PDF). Inorganic Chemistry. 7 (2): 389–390. doi:10.1021/ic50060a047.
- ↑ Jobic, S.; Deniard, P.; Brec, R.; Rouxel, J.; Drew, M. G. B.; David, W. I. F. (1990). "Properties of the transition metal dichalcogenides: the case of IrS2 and IrSe2". Journal of Solid State Chemistry. 89 (2): 315–327. Bibcode:1990JSSCh..89..315J. doi:10.1016/0022-4596(90)90273-Z.
- ↑ Vaughan, David J.; Craig, James R. (1978). Mineral chemistry of metal sulfides. Cambridge Earth Science Series. Cambridge: Cambridge University Press. ISBN 0521214890.
- ↑ Chianelli, R. R.; Berhault, G.; Raybaud, P.; Kasztelan, S.; Hafner, J.; Toulhoat, H. (8 March 2002). "Periodic trends in hydrodesulfurization: in support of the Sabatier principle". Applied Catalysis A: General. 227 (1–2): 83–96. doi:10.1016/S0926-860X(01)00924-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.