 | |
| Clinical data | |
|---|---|
| Trade names | Lopid, Jezil, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a686002 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Close to 100% |
| Protein binding | 95% |
| Metabolism | Liver (CYP3A4) |
| Elimination half-life | 1.5 hours |
| Excretion | Kidney 94% Feces 6% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.042.968 |
| Chemical and physical data | |
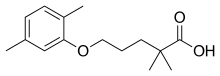
| Formula | C15H22O3 |
| Molar mass | 250.338 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 61 to 63 °C (142 to 145 °F) |
| |
| |
| (verify) | |
Gemfibrozil, sold under the brand name Lopid among others, is a medication used to treat abnormal blood lipid levels.[2] It is generally less preferred than statins.[2][3] Use is recommended together with dietary changes and exercise.[2] It is unclear if it changes the risk of heart disease.[2] It is taken by mouth.[2]
Common side effects include headache, dizziness, feeling tired, and intestinal upset.[2] Serious side effects may include angioedema, gallstones, liver problems, and muscle breakdown.[2] Use in pregnancy and breastfeeding is of unclear safety.[4] It belongs to the fibrates group of medications and works by decreasing the breakdown of lipids in fat cells.[2]
Gemfibrozil was patented in 1968, and came into medical use in 1982.[5] It is available as a generic medication.[3] In 2020, it was the 189th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[6][7]
Medical uses
- Hyperlipidemia (Type III)
- Hypertriglyceridemia (Type IV): Gemfibrozil, though not as effective as niacin (nicotinic acid, a form of Vitamin B3), is better tolerated.
- Reduce triglyceride levels [8]
- Reduce very low density lipoprotein (VLDL) levels
- Modest reduction of low density lipoprotein (LDL) levels
- Moderate increase in high density lipoprotein (HDL) levels
Side effects
- GI distress
- Musculoskeletal pain
- Increased incidence of gallstone
- Hypokalemia (low blood potassium)
- Increased risk of cancer
Contraindications
- Gemfibrozil should not be given to these patients:
- Hepatic dysfunction
- Gemfibrozil should be used with caution in these higher risk categories:
- Biliary tract disease
- Renal dysfunction
- Pregnant women
- Obese patients
Drug interactions
- Anticoagulants: Gemfibrozil potentiates the action of warfarin and indanedione anticoagulants.
- Statin drugs: Concomitant administration of fibrates (including gemfibrozil) with statin drugs increases the risk of muscle cramping, myopathy, and rhabdomyolysis.[9]
- Gemfibrozil inhibits the activation of the liver's Cytochrome P450 system and CYP2C8, reducing hepatic metabolism of many drugs, and prolonging their half lives and duration of action.
- Drugs metabolized by the Cytochrome P450 system include:
- Many antidepressants
- Many antipsychotics
- Many antiepileptics
- Theophylline and other methylxanthine drugs
- Several anesthetic agents
- Oral contraceptive pills
- Statins
- Warfarin
- Selexipag
- Drugs metabolized by the Cytochrome P450 system include:
Mechanism of actions
The exact mechanism of action of gemfibrozil is unknown; however, several theories exist regarding the very low density lipoprotein (VLDL) effect; it can inhibit lipolysis and decrease subsequent hepatic fatty acid uptake as well as inhibit hepatic secretion of VLDL; together these actions decrease serum VLDL levels and increase HDL-cholesterol; the mechanism behind HDL elevation is currently unknown.
Gemfibrozil increases the activity of extrahepatic lipoprotein lipase (LL), thereby increasing lipoprotein triglyceride lipolysis. It does so by activating peroxisome proliferator-activated receptor alpha (PPARα) 'transcription factor ligand', a receptor that is involved in metabolism of carbohydrates and fats, as well as adipose tissue differentiation. This increase in the synthesis of lipoprotein lipase thereby increases the clearance of triglycerides. Chylomicrons are degraded, VLDLs are converted to LDLs, and LDLs are converted to HDL. This is accompanied by a slight increase in secretion of lipids into the bile and ultimately the intestine. Gemfibrozil also inhibits the synthesis and increases the clearance of apolipoprotein B, a carrier molecule for VLDL.[10]
History
Gemfibrozil was selected from a series of related compounds synthesized in the laboratories of the American company Parke-Davis in the late 1970s. It came from research for compounds that lower plasma lipid levels in humans and in animals.[11]
Environmental data
Gemfibrozil has been detected in biosolids (the solids remaining after sewage treatment) at concentrations up to 2650 ng/g wet weight.[12] This indicates that it survives the wastewater treatment process. It is also detected as environmental persistent micropollutant in aquifers and in groundwaters in karstic areas.[13]
References
- ↑ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- 1 2 3 4 5 6 7 8 "Gemfibrozil Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- 1 2 British National Formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 198–199. ISBN 9780857113382.
- ↑ "Gemfibrozil Use During Pregnancy". Drugs.com. Retrieved 3 March 2019.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 474. ISBN 9783527607495.
- ↑ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ↑ "Gemfibrozil - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- ↑ "Gemfibrozil". WebMD.com. Retrieved 14 June 2014.
- ↑ "Medicines Complete". Medicines Complete. British National Formulary. Retrieved 1 February 2020.
- ↑ "Gemfibrozil". PubChem. U.S. National Library of Medicine.
- ↑ Rodney G, Uhlendorf P, Maxwell RE (1976). "The hypolipidaemic effect of gemfibrozil (CI-719) in laboratory animals". Proceedings of the Royal Society of Medicine. 69 (2_suppl): 6–10. doi:10.1177/00359157760690S203. PMC 1864017. PMID 828263.
- ↑ "Biosolids". U.S. Environmental Protection Agency. 23 April 2014.
- ↑ Doummar J, Aoun M (August 2018). "Assessment of the origin and transport of four selected emerging micropollutants sucralose, Acesulfame-K, gemfibrozil, and iohexol in a karst spring during a multi-event spring response". Journal of Contaminant Hydrology. 215: 11–20. Bibcode:2018JCHyd.215...11D. doi:10.1016/j.jconhyd.2018.06.003. PMID 29983209. S2CID 51599602.
Further reading
- Bellosta S, Paoletti R, Corsini A (June 2004). "Safety of statins: focus on clinical pharmacokinetics and drug interactions". Circulation. 109 (23 Suppl 1): III50-7. doi:10.1161/01.cir.0000131519.15067.1f. hdl:2434/143653. PMID 15198967.
- Quintanilla Rodriguez BS, Correa R (2020). "Gemfibrozil". StatPearls. National Center for Biotechnology Information (NCBI). PMID 31424850. Bookshelf ID: NBK545266.
External links
- "Gemfibrozil". Drug Information Portal. U.S. National Library of Medicine.
- "Lopid International Study" (PDF). European Medicines Agency. Archived from the original (PDF) on 11 June 2007.
- "Indian Health Service National Pharmacy and Therapeutics Committee Review of Statins, Fibrates, and Niacin" (PDF). Indian Health Service. San Diego: U.S. Department of Health and Human Services. 12–13 February 2009. Archived from the original (PDF) on 22 June 2014.