 | |
| Clinical data | |
|---|---|
| Trade names | Eovist |
| Other names | Gadoxetate disodium (USAN US) |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
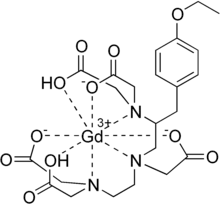
| Formula | C23H30GdN3O11 |
| Molar mass | 681.75 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Gadoxetic acid is a gadolinium-based MRI contrast agent.[3] Its salt, gadoxetate disodium, is marketed as Primovist in Europe and Eovist in the United States by Bayer HealthCare Pharmaceuticals.[1][4]
Medical uses
It is used to increase the T1 signal intensity while imaging the liver lesions such as benign cysts, haemangioma, and liver cancer. It is excreted into bile by active secretion.[5]
Pharmacokinetics
In those with end-stage renal failure, the clearance rate is only 17% with terminal half-life of 12 times longer than those with normal renal function.[5]
See also
References
- 1 2 "Eovist- gadoxetate disodium injection, solution". DailyMed. National Library of Medicine, National Institutes of Health, U.S. Health & Human Services. Retrieved 21 April 2021.
- ↑ "Active substance(s): gadoxetic acid disodium" (PDF). List of nationally authorised medicinal products. European Medicines Agency. 14 January 2021.
- ↑ Koh DM, Ba-Ssalamah A, Brancatelli G, Fananapazir G, Fiel MI, Goshima S, et al. (August 2021). "Consensus report from the 9th International Forum for Liver Magnetic Resonance Imaging: applications of gadoxetic acid-enhanced imaging". European Radiology. 31 (8): 5615–5628. doi:10.1007/s00330-020-07637-4. PMC 8270799. PMID 33523304.
- ↑ "Eovist - Homepage". Archived from the original on 2009-04-25. Retrieved 2009-03-22.
- 1 2 "Clinical pharmacology and biopharmaceutics review" (PDF). Center for drug evaluation and research. Archived from the original (PDF) on 12 May 2021. Retrieved 15 December 2022.
External links
- "Gadoxetic acid". Drug Information Portal. U.S. National Library of Medicine.
- "Gadoxetate disodium". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.