| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Thiirane | |||
| Systematic IUPAC name
Thiacyclopropane | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 102379 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.359 | ||
| EC Number |
| ||
| 1278 | |||
| KEGG | |||
| MeSH | ethylene+sulfide | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1992 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H4S | |||
| Molar mass | 60.11 g·mol−1 | ||
| Appearance | Pale, yellow liquid | ||
| Density | 1.01 g cm−3 | ||
| Melting point | −109 °C (−164 °F; 164 K) | ||
| Boiling point | 56 °C; 133 °F; 329 K | ||
| Vapor pressure | 28.6 kPa (at 20 °C) | ||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH⦵298) |
51-53 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
-2.0126 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Danger | |||
| H225, H301, H318, H331 | |||
| P210, P261, P280, P301+P310, P305+P351+P338, P311 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 10 °C (50 °F; 283 K) | ||
| Related compounds | |||
Related heterocycles |
Ethylene oxide Aziridine Borirane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
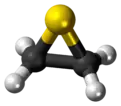
Thiirane, more commonly known as ethylene sulfide, is the cyclic chemical compound with the formula C2H4S.[2] It is the smallest sulfur-containing heterocycle and the simplest episulfide. Like many organosulfur compounds, this species has a highly unpleasant odour. Thiirane is also used to describe any derivative of the parent ethylene sulfide.
Structure
According to electron diffraction, the C-C and C-S distances in ethylene sulfide are respectively 1.473 and 1.811 Å. The C-C-S and C-S-C angles are respectively 66.0 and 48.0°.[3]
Preparation and reactions
It can be prepared by the reaction of ethylene carbonate and KSCN.[4] For this purpose the KSCN is first melted under vacuum to remove water.
- KSCN + C2H4O2CO → KOCN + C2H4S + CO2
Ethylenesulfide adds to amines to afford 2-mercaptoethylamines,[5] which are good chelating ligands.
- C2H4S + R2NH → R2NCH2CH2SH
This process is often called mercaptoethylation.[6]
Oxidation of thiirane with periodate gives ethylene episulfoxide.
References
- 1 2 "thiirane (CHEBI:30977)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- ↑ Warren Chew; David N. Harpp (1993). "Recent aspects of thiirane chemistry". Journal of Sulfur Chemistry. 15 (1): 1–39. doi:10.1080/01961779308050628.
- ↑ Wataru Ando; Nami Choi; Norihiro Tokitoh (1996). "Thiiranes and Thiirenes: Monocyclic". Comprehensive Heterocyclic Chemistry II. Vol. 1A. pp. 173–240. doi:10.1016/B978-008096518-5.00005-8. ISBN 978-0-08-096518-5.
- ↑ Searles, S.; Lutz, E. F.; Hays, H. R.; Mortensen, H. E. (1962). "Ethylene Sulfide". Organic Syntheses. 42: 59. doi:10.15227/orgsyn.042.0059.
- ↑ R. J. Cremlyn "An Introduction to Organosulfur Chemistry" John Wiley and Sons: Chichester (1996). ISBN 0-471-95512-4.
- ↑ Gunars Zelans; Jacquelyn Gervay-Hague; Ivy Maulie (2010). "Ethylene Sulfide". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.re079.pub2. ISBN 978-0-471-93623-7.