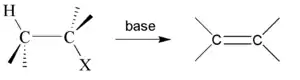
In chemistry, dehydrohalogenation is an elimination reaction which removes a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications.
Dehydrohalogenation from alkyl halides
Traditionally, alkyl halides are substrates for dehydrohalogenations. The alkyl halide must be able to form an alkene, thus halides having no C–H bond on an adjacent carbon are not suitable substrates. Aryl halides are also unsuitable. Upon treatment with strong base, chlorobenzene dehydrohalogenates to give phenol via a benzyne intermediate.
Base-promoted reactions to alkenes
When treated with a strong base many alkyl chlorides convert to corresponding alkene.[1] It is also called a β-elimination reaction and is a type of elimination reaction. Some prototypes are shown below:
Here ethyl chloride reacts with potassium hydroxide, typically in a solvent such as ethanol, giving ethylene. Likewise, 1-chloropropane and 2-chloropropane give propene.
Zaitsev's rule helps to predict regioselectivity for this reaction type.
In general, the reaction of a haloalkane with potassium hydroxide can compete with an SN2 nucleophilic substitution reaction by OH− a strong, unhindered nucleophile. Alcohols are however generally minor products. Dehydrohalogenations often employ strong bases such as potassium tert-butoxide (K+ [CH3]3CO−).
Base-promoted reactions to alkynes
Upon treatment with strong base, vicinal dihalides convert to alkynes.[2]
Thermal cracking
On an industrial scale, base-promoted dehydrohalogenations as described above are disfavored. The disposal of the alkali halide salt is problematic. Instead thermally-induced dehydrohalogenations are preferred. One example is provided by the production of vinyl chloride by heating 1,2-dichloroethane:[3]
- CH2Cl-CH2Cl → CH2=CHCl + HCl
The resulting HCl can be reused in oxychlorination reaction.
Thermally induced dehydrofluorinations are employed in the production of fluoroolefins and hydrofluoroolefins. One example is the preparation of 1,2,3,3,3-pentafluoropropene from 1,1,2,3,3,3-hexafluoropropane:
- CF2HCH(F)CF3 → CHF=C(F)CF3 + HF
Other dehydrohalogenations
Epoxides
Chlorohydrins, compounds with the connectivity R(HO)CH-CH(Cl)R', undergo dehydrochlorination to give epoxides. This reaction is employed industrially to produce millions of tons of propylene oxide annually from propylene chlorohydrin:[4]
- CH3CH(OH)CH2Cl + KOH → CH3CH(O)CH2 + H2O + KCl
Isocyanides
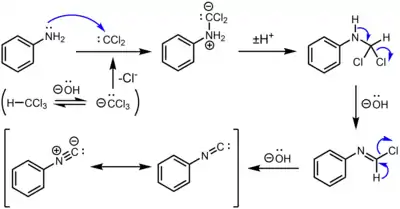
The carbylamine reaction for the synthesis of isocyanides from the action of chloroform on a primary amine involves three dehydrohalogenations. The first dehydrohalogenation is the formation of dichlorocarbene:
- KOH + CHCl3 → KCl + H2O + CCl2
Two successive base-mediated dehydrochlorination steps result in formation of the isocyanide.[5]
Coordination compounds
Dehydrohalogenation is not limited to organic chemistry. Some metal-organic coordination compounds can eliminate hydrogen halides,[6] either spontaneously,[7] thermally, or by mechanochemical reaction with a solid base such as potassium hydroxide.[8]
For example, salts that contain acidic cations hydrogen bonded to halometallate anions will often undergo dehydrohalogenation reactions reversibly:[6]
- [B–H]+···[X–MLn]− ⇌ [B–MLn] + HX
where B is a basic ligand such as a pyridine, X is a halogen (typically chlorine or bromine), M is a metal such as cobalt, copper, zinc, palladium or platinum, and Ln are spectator ligands.
References
- ↑ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595
- ↑ A. Le Coq and A. Gorgues (1979). "Alkyness via Phase Transfer-Catalyzed Dehydrohalogenation: Propiolaldehyde Diethyl Acetal". Organic Syntheses. 59: 10. doi:10.15227/orgsyn.059.0010.
- ↑ M. Rossberg et al. "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2
- ↑ Nijhuis, T. Alexander; Makkee, Michiel; Moulijn, Jacob A.; Weckhuysen, Bert M. "The Production of Propene Oxide: Catalytic Processes and Recent Developments" Industrial & Engineering Chemistry Research 2006, volume 45, 3447-3459. doi:10.1021/ie0513090
- ↑ Gokel, G.W.; Widera, R.P.; Weber, W.P. (1988). "Phase-transfer Hofmann carbylamine reaction: tert-butyl isocyanide". Organic Syntheses. 55: 232. doi:10.15227/orgsyn.055.0096.
- 1 2 Martí-Rujas, Javier; Guo, Fang (2021). "Dehydrohalogenation reactions in second-sphere coordination complexes". Dalton Trans. 50 (34): 11665–11680. doi:10.1039/D1DT02099D. PMID 34323900. S2CID 236496267.
- ↑ Mínguez Espallargas, Guillermo; Brammer, Lee; van de Streek, Jacco; Shankland, Kenneth; Florence, Alastair J.; Adams, Harry (2006). "Reversible Extrusion and Uptake of HCl Molecules by Crystalline Solids Involving Coordination Bond Cleavage and Formation". J. Am. Chem. Soc. 128 (30): 9584–9585. doi:10.1021/ja0625733. PMID 16866484.
- ↑ James, Stuart L.; Adams, Christopher J.; Bolm, Carsten; Braga, Dario; Collier, Paul; Friščić, Tomislav; Grepioni, Fabrizia; Harris, Kenneth D. M.; Hyett, Geoff; Jones, William; Krebs, Anke; Mack, James; Maini, Lucia; Orpen, A. Guy; Parkin, Ivan P.; Shearouse, William C.; Steed, Jonathan W.; Waddell, Daniel C. (2012). "Mechanochemistry: opportunities for new and cleaner synthesis" (PDF). Chem. Soc. Rev. 41 (1): 413–447. doi:10.1039/C1CS15171A. PMID 21892512.
External links
- Dehydrohalogenation of Alkyl Halides Archived 2021-04-11 at the Wayback Machine