 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Pharmacokinetic data | |
| Bioavailability | Moderate |
| Protein binding | <25% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.429 |
| Chemical and physical data | |
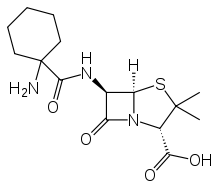
| Formula | C15H23N3O4S |
| Molar mass | 341.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ciclacillin (INN) or cyclacillin (USAN), trade names Cyclapen, Cyclapen-W, Vastcillin, and others, is an aminopenicillin antibiotic. Its spectrum of activity is similar to that of ampicillin, although it is less susceptible to beta-lactamases than ampicillin and has much higher bioavailability.[1] A large randomized, double-blind clinical trial published in 1978 also showed that ciclacillin is associated with significantly fewer and milder adverse effects than ampicillin;[2] later studies seemed to confirm this improved tolerability, at least in children.[3][4]
Ciclacillin has been superseded by newer antibiotics and is no longer in clinical use, at least in the United States.[5]
Synthesis
In an attempt to form orally active penicillins unrelated to ampicillin, use was made of the fact that certain spiro α-amino acids, such as Cycloleucine, are well absorbed orally and transported like normal amino acids.
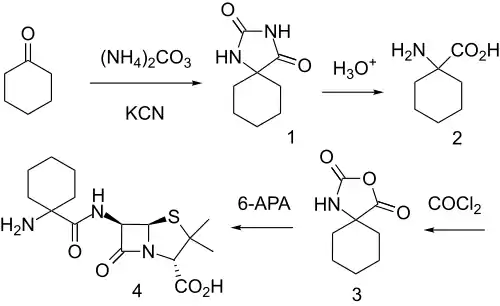
Reaction of cyclohexanone with ammonium carbonate and KCN under the conditions of the Bucherer-Bergs reaction led to hydantoin 1. On acid hydrolysis, α-amino acid 2 resulted. Treatment with phosgene both protected the amino group and activated the carboxyl group toward amide formation (as 3) and reaction with 6-aminopenicillanic acid (6-APA) gave cyclacillin (4).
This artifice seems to have worked, since cyclacillin is more active in vivo than its in vitro spectrum suggests.
References
- ↑ Warren GH (1976). "Cyclacillin: microbiological and pharmacological properties and use in chemotherapy of infection - a critical appraisal". Chemotherapy. 22 (3–4): 154–182. doi:10.1159/000221924. PMID 773605.
- ↑ Gold JA, Hegarty CP, Deitch MW, Walker BR (January 1979). "Double-blind clinical trials of oral cyclacillin and ampicillin". Antimicrobial Agents and Chemotherapy. 15 (1): 55–58. doi:10.1128/aac.15.1.55. PMC 352600. PMID 371540.
- ↑ McLinn SE, Goldberg F, Kramer R, Saltstein E, Bomze JP, Deitch MW (October 1982). "Double-blind multicenter comparison of cyclacillin and amoxicillin for the treatment of acute otitis media". The Journal of Pediatrics. 101 (4): 617–621. doi:10.1016/S0022-3476(82)80724-5. PMID 6750067.
- ↑ McLinn SE, Kaplan J, West N (1983). "Multicenter comparison of cyclacillin and amoxicillin in the treatment of acute streptococcal pharyngitis". Clinical Therapeutics. 5 (3): 299–304. PMID 6342785.
- ↑ Gorbach SL, Bartlett JG, Blacklow NR (2004). Infectious diseases (3rd ed.). Hagerstown, MD: Lippincott Williams & Wilkins. p. 186. ISBN 0-7817-3371-5. Retrieved on September 7, 2008 through Google Book Search.
- ↑ Alburn HE, Clark RE, Fletcher H, Grant NH (1967). "Synthesis of new broad-spectrum aminoalicyclic penicillins". Antimicrobial Agents and Chemotherapy. 7: 586–589. PMID 5596194.
Further reading
- Scheld WM, Sydnor A, Farr B, Gratz JC, Gwaltney JM (September 1986). "Comparison of cyclacillin and amoxicillin for therapy for acute maxillary sinusitis". Antimicrobial Agents and Chemotherapy. 30 (3): 350–353. doi:10.1128/aac.30.3.350. PMC 180557. PMID 3535660.