 | |
| Clinical data | |
|---|---|
| Trade names | Aramine, Metaramin, Pressonex |
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, endotracheal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | ~45% |
| Metabolism | Liver |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
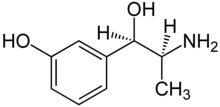
| Formula | C9H13NO2 |
| Molar mass | 167.208 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Metaraminol, previously sold under the brand name Aramine among others and also known as metaradrine, is a stereoisomer of meta-hydroxynorephedrine (3,β-dihydroxyamphetamine), is a potent sympathomimetic amine used in the prevention and treatment of hypotension, particularly as a complication of anesthesia. It is an α1-adrenergic receptor agonist with some β-adrenergic effect.[2] It is currently sold in its generic form by Slayback Pharma.[3]
Pharmacology and use as a vasopressor
Metaraminol is given intravenously as either a bolus (often 0.5–1 mg doses) or as an infusion, usually via peripheral intravenous access. Metaraminol is commonly available as 10 mg in 1 mL, that requires dilution prior to administration (often made up to a 0.5 mg/mL solution), however pre-prepared syringes of metaraminol for bolus use for hypotension are also commonly available.[4][5]
Pharmacodynamics
The dominant mechanism for the vasopressor action of metaraminol is indirect,[6] with metaraminol displacing noradrenaline from neuronal vesicles in order for the noradrenaline to exert its vasopressor action.[7] Metaraminol at higher doses may have direct alpha-adrenergic agonist and β1 adrenergic agonist effects.[6] However at doses common in clinical practice, the indirect α1 adrenergic effects predominate, such that reflex bradycardia is a common side-effect.
Research
Metaraminol is also used in the treatment of priapism.[8][9][10]
References
- ↑ "Injection : Aramine (Metaraminol Bitartrate)" (PDF). U.S. Food and Drug Administration (FDA). Retrieved 12 March 2022.
- ↑ Kee VR (August 2003). "Hemodynamic pharmacology of intravenous vasopressors". Crit Care Nurse. 23 (4): 79–82. doi:10.4037/ccn2003.23.4.79. PMID 12961786.
- ↑ "ANDA Approval for Metaraminol" (PDF). United States Food and Drug Administration. 24 August 2021. Retrieved 13 August 2022.
- ↑ "Metaraminol 0.5 mg/ml, Solution for Injection in pre-filled syringe - Summary of Product Characteristics (SmPC) - (emc)". www.medicines.org.uk. Retrieved 19 October 2022.
- ↑ Goodrick N, Wentrup T, Messer G, Gleeson P, Culwick M, Goulding G (21 August 2020). "Pre-filled emergency drugs: The introduction of pre-filled metaraminol and ephedrine syringes into the main operating theatres of a major metropolitan centre". Australasian Anaesthesia (2013): 127–134.
- 1 2 "Metaraminol". Deranged Physiology. Retrieved 19 October 2022.
- ↑ Harrison DC, Chidsey CA, Braunwald E (September 1963). "Studies on the Mechanism of Action of Metaraminol (Aramine)". Annals of Internal Medicine. 59 (3): 297–305. doi:10.7326/0003-4819-59-3-297. PMID 14065947.
- ↑ McDonald M, Santucci R (2004). "Successful management of stuttering priapism using home self-injections of the alpha-agonist metaraminol". Int Braz J Urol. 30 (2): 121–2. doi:10.1590/S1677-55382004000200007. PMID 15703094.
- ↑ Koga S, Shiraishi K, Saito Y (1990). "Post-traumatic priapism treated with metaraminol bitartrate: case report". J Trauma. 30 (12): 1591–3. doi:10.1097/00005373-199012000-00029. PMID 2258979.
- ↑ Block T, Sturm W, Ernst G, Staehler G, Schmiedt E (1988). "[Metaraminol in therapy of various forms of priapism]". Urologe A. 27 (4): 225–9. PMID 3140463.
External links
- "Metaraminol". Drug Information Portal. U.S. National Library of Medicine.
- "Metaraminol bitartrate". Drug Information Portal. U.S. National Library of Medicine.