 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Albenza, Valbazen, Zentel, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610019 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | <5%[2] |
| Protein binding | 70%[2] |
| Metabolism | Hepatic[2] |
| Elimination half-life | 8-12 hours[2] |
| Excretion | Bile (humans) Urine (ruminants) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.053.995 |
| Chemical and physical data | |
| Formula | C12H15N3O2S |
| Molar mass | 265.33 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 208 to 210 °C (406 to 410 °F) |
| |
| |
| (verify) | |
Albendazole is a broad-spectrum anthelmintic and antiprotozoal agent of the benzimidazole type.[3] It is used for the treatment of a variety of intestinal parasite infections, including ascariasis, pinworm infection, hookworm infection, trichuriasis, strongyloidiasis, taeniasis, clonorchiasis, opisthorchiasis, cutaneous larva migrans, giardiasis, and gnathostomiasis, among other diseases.[3]
Common side effects include nausea, abdominal pain, and headache.[3] Rare but potentially serious side effects include bone marrow suppression which usually improves on discontinuing the medication. Liver inflammation has been reported and those with prior liver problems are at greater risk.[3] It is pregnancy category D in Australia, meaning it may cause harm if taken by pregnant women.[3][4]
Albendazole was developed in 1975.[5] It is on the World Health Organization's List of Essential Medicines.[6]
Medical uses
Albendazole is an effective treatment for:
- Flatworms
- Fasciolosis[3]
- Cestodes (tapeworms), as an alternative to praziquantel or niclosamide for adult beef tapeworms and as an alternative to praziquantel for pork tapeworms.[7] It is also given for infections by T. crassiceps.[8] Though praziquantel is often better at treating tapeworm infections, albendazole is used more often in endemic countries due to being cheaper and having a broader spectrum.[9]
- Cysticercosis[10] (especially neurocysticercosis), which is caused by the larval form of the pork tapeworm[3] (i.e. albendazole is the drug of choice for larval pork tapeworms, but not adult pork tapeworms).[7] Old cysts are not affected.[9]
- Echinococcosis[10][11] of the liver, lung, and peritoneum (caused by the larval form of the dog tapeworm, or of the alveoli (caused by E. multilocularis) when surgical excision is not possible.[3] Alveolar and cystic echinococcosis may require lifelong treatment with albendazole, which only prevents the parasites from growing and reproducing rather than killing them.[12]
- Nematodes
- Ascariasis, which can be cured with a single dose of albendazole.[13][14]
- Baylisascariasis, caused by the raccoon roundworm. Albendazole can achieve good results (95%-100% efficacy after a 10-day course of treatment) if treatment is initiated within 72 hours of ingestion of the egg-containing raccoon feces.[15] Corticosteroids are sometimes added in cases of eye and CNS infections.[3]
- Pinworm infection[13]
- Filariasis; since albendazole's disintegration of the microfilariae ("pre-larva") can cause an allergic reaction, antihistamines or corticosteroids are sometimes added to treatment. In cases of lymphatic filariasis (elephantiasis) caused by Wuchereria bancrofti or Brugia malayi, albendazole is sometimes given as an adjunct to ivermectin or diethylcarbamazine in order to suppress microfilaremia. It can also be given for Loa loa filariasis as an adjunct or replacement to diethylcarbamazine.[3][9] Albendazole has an embryotoxic effect on Loa loa adults and thus slowly reduces microfilaremia.[14]
- Gnathostomiasis when caused by Gnathostoma spinigerum.[3] Albendazole has a similar effectiveness to ivermectin in these cases, though it needs to be given for 21 days rather than the 2 days needed for ivermectin.[12]
- Gongylonemiasis[3]
- Hookworm infections,[13] including cutaneous larva migrans caused by hookworms of genus Ancylostoma. A single dose of albendazole is sufficient to treat intestinal infestations by A. duodenale or Necator americanus.[3][14]
- Intestinal capillariasis,[13] as an alternative to mebendazole[3]
- Mansonelliasis when caused by Mansonella perstans. Albendazole is effective against adult worms but not against the immature microfilariae.[12]
- Oesophagostomumiasis, when caused by Oesophagostomum bifurcum[3]
- Strongyloidiasis,[13] as an alternative to ivermectin or thiabendazole.[3][16] Albendazole can be given with diethylcarbamazine to lower microfilaremia levels.[14]
- Toxocariasis, also called "visceral larva migrans", when caused by the dog roundworm Toxocara canis or cat roundworm T. catis. Corticosteroids can be added in severe cases, and surgery might be required to repair secondary damage.[3]
- Trichinosis, when caused by Trichinella spiralis[7] or T. pseudospiralis. Albendazole has a similar efficacy to thiabendazole, but fewer side effects.[12] It works best when given early, acting on the adult worms in the intestine before they generate larva that can penetrate the muscle and cause a more widespread infection. Corticosteroids are sometimes added on to prevent inflammation caused by dying larva.[9]
- Trichostrongyliasis, as an alternative to pyrantel pamoate.[3][13] A single dose is sufficient for treatment.[9]
- Trichuriasis (whipworm infection),[13] sometimes considered as an alternative to mebendazole[3][7] and sometimes considered to be the drug of choice. Only a single dose of albendazole is needed.[14] It can also be given with ivermectin.[17]
- Giardiasis, as an alternative or adjunct to metronidazole, especially in children[3][18]
- Microsporidiosis, including ocular microsporidiosis caused by Encephalitozoon hellem or E. cuniculi, when combined with topical fumagillin[3][18]
- Granulomatous amoebic encephalitis, when caused by the amoeba Balamuthia mandrillaris, in combination with miltefosine and fluconazole[8]
- Arthropods
- Crusted scabies, when combined with topical crotamiton and salicylic acid[8]
- Head lice infestation, though ivermectin is much better[8]
- Intestinal myiasis[19]
Though albendazole is effective in treating many diseases, it is only FDA-approved for treating hydatid disease caused by dog tapeworm larvae and neurocysticercosis caused by pork tapeworm larvae.[20]
Pregnancy
Albendazole is a pregnancy class D drug in Australia. It is contraindicated in the first trimester of pregnancy, and should be avoided up to one month before conception. While studies in pregnant rats and rabbits have shown albendazole to be teratogenic,[21][22] albendazole has been found to be safe in humans during the second and third trimesters.[23][24] It can, however, possibly cause infantile eczema when given during pregnancy.[25]
In pregnant dogs, albendazole use has led to puppies with reduced weight and with cleft palates. Birds have lower rates of laying eggs and hatching when given albendazole.[26]
Albendazole sulfoxide is secreted into breast milk at around 1.5% of the maternal dose, though oral absorption is poor enough that it is unlikely to affect nursing infants.[21][27]
Contraindications
Hypersensitivity to the benzimidazole class of compounds contraindicates its use.[13]
Side effects
The most common side effects of albendazole are experienced by over 10% of people and include headache and abnormal liver function.[2] Elevation of liver enzymes occurs in 16% of patients receiving treatment specifically for hydatid disease and goes away when treatment ends.[9][28] Liver enzymes usually increase to two to four times the normal levels (a mild to moderate increase).[29] An estimated 1–10% of people experience abdominal pain, nausea or vomiting, dizziness or vertigo, increased intracranial pressure, meningeal signs, temporary hair loss, and fever. The headache, nausea, and vomiting are thought to be caused by the sudden destruction of cysticerci (tapeworm larvae), which causes acute inflammation.[30] Fewer than 1% of people get hypersensitivity reactions such as rashes and hives, leukopenias (drop in white blood cell levels) such as agranulocytosis and granulocytopenia, thrombocytopenia (reduced platelet count), pancytopenia (drop in white blood cells, red blood cells, and platelets), hepatitis, acute liver failure, acute kidney injury, irreversible bone marrow suppression, and aplastic anemia.[2][31]
Side effects can be different when treating for hydatid disease versus neurocysticercosis: for example, those being treated for the former are more likely to experience elevated liver enzymes and abdominal pain, while those being treated for the latter are more likely to experience headache.[28] Treating hydatid disease can also unmask undiagnosed neurocysticercosis.[28] People receiving albendazole for the treatment of neurocysticercosis can have neurological side effects such as seizures, increased intracranial pressure, and focal signs caused by the inflammatory reaction that occurs when parasites in the brain are killed. Steroids and anticonvulsants are often given with albendazole when treating neurocysticercosis to avoid these effects.[28] Those being treated for retinal neurocysticercosis can face retinal damage if they are not first checked for ocular cysticeri, since changes to existing lesions in the eye by albendazole can cause permanent blindness.[9]
Overdose
Because of its low solubility, albendazole often cannot be absorbed in high enough quantities to be toxic.[30] The oral LD50 of albendazole in rats was found to be 2,500 mg/kg.[22] It takes 20 times the normal dose to kill a sheep, and 30 times the normal dose to kill cattle.[1] Overdose affects the liver, testicles, and GI tract the most. It can manifest with lethargy, loss of appetite, vomiting, diarrhea, intestinal cramps, dizziness, convulsions, and sleepiness. There is no specified antidote.[26]
Interactions
The antiepileptics carbamazepine, phenytoin, and phenobarbital lower the plasma concentration and half-life of albendazole sulfoxide's R(+) enantiomer.[32]
| Drug | Change in AUC | Change in Cmax |
|---|---|---|
| Carbamazepine | 49% decrease | 50–63% decrease |
| Phenobarbitol | 61% decrease | 50–63% decrease |
| Phenytoin | 66% decrease | 50–63% decrease |
The antacid cimetidine heightens serum albendazole concentrations, increases the half-life of albendazole, and doubles albendazole sulfoxide levels in bile.[33][28] It was originally thought to work by increasing albendazole bioavailability directly; however, it is now known that cimetidine inhibits the breakdown of albendazole sulfoxide by interfering with CYP3A4.[12] The half-life of albendazole sulfoxide thus increases from 7.4 hours to 19 hours.[34] This might be a helpful interaction on more severe cases, because it boosts the potency of albendazole.[35] Paradoxically, cimetidine also inhibits the absorption of albendazole by reducing gastric acidity.[34]
Several other interactions exist. Corticosteroids increase the steady-state plasma concentration of albendazole sulfoxide;[9] dexamethasone, for example, can increase the concentration by 56% by inhibiting the elimination of albendazole sulfoxide.[28][30] The anti-parasitic praziquantel increases the maximum plasma concentration of albendazole sulfoxide by 50%,[28] and the anti-parasitic levamisole increases the AUC (total drug exposure) by 75%.[17] Grapefruit inhibits the metabolism of albendazole within the intestinal mucosa. Finally, long-term administration of the antiretroviral ritonavir, which works as a CYP3A4 inhibitor, decreases the maximum concentration of albendazole in the plasma as well as the AUC.[34]
Pharmacology
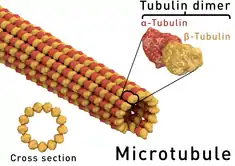
Mechanism of action
As a vermicide, albendazole causes degenerative alterations in the intestinal cells of the worm by binding to the colchicine-sensitive site of β-tubulin, thus inhibiting its polymerization or assembly into microtubules (it binds much better to the β-tubulin of parasites than that of mammals).[3][28] Albendazole leads to impaired uptake of glucose by the larval and adult stages of the susceptible parasites, and depletes their glycogen stores. Albendazole also prevents the formation of spindle fibers needed for cell division, which in turn blocks egg production and development; existing eggs are prevented from hatching.[12][36] Cell motility, maintenance of cell shape, and intracellular transport are also disrupted.[37] At higher concentrations, it disrupts the helminths' metabolic pathways by inhibiting metabolic enzymes such as malate dehydrogenase and fumarate reductase, with inhibition of the latter leading to less energy produced by the Krebs cycle.[1][26][38] Due to diminished ATP production, the parasite is immobilized and eventually dies.
Some parasites have evolved some resistance to albendazole by having a different set of acids constituting β-tubulin, decreasing the binding affinity of albendazole.[28] Some parasites (especially filarial nematodes) live in symbiosis with Wolbachia, a type of intracellular parasite bacteria. In such cases the Wolbachia are necessary to the survival of the parasitic worms.[39] Elimination of Wolbachia from these filarial nematodes generally results in either death or sterility of the host nematode.[40]
Pharmacokinetics
Oral absorption of albendazole varies among species, with 1–5% of the drug being successfully absorbed in humans, 20–30% in rats, and 50% in cattle.[41]
The absorption also largely depends on gastric pH. People have varying gastric pHs on empty stomachs, and thus absorption from one person to another can vary wildly when taken without food.[16] Generally, the absorption in the GI tract is poor due to albendazole's low solubility in water.[3] It is, however, better absorbed than other benzimidazole carbamates.[17] Food stimulates gastric acid secretion, lowering the pH and making albendazole more soluble and thus more easily absorbed.[34] Oral absorption is especially increased with a fatty meal, as albendazole dissolves better in lipids, allowing it to cross the lipid barrier created by the mucus surface of the GI tract.[37][41] To target intestinal parasites, albendazole is taken on an empty stomach to stay within the gut.[42]
Absorption is also affected by how much of the albendazole is degraded within the small intestine by metabolic enzymes in the villi.[16]
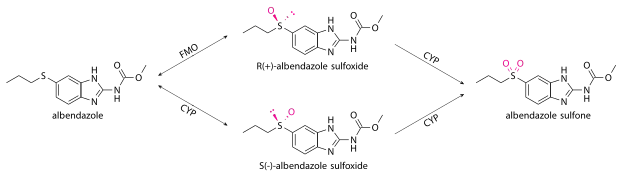
The pharmacokinetics of albendazole differ slightly between men and women: women have a lower oral clearance and volume of distribution, while men have a lower serum peak concentration.[34]
Albendazole undergoes very fast first-pass metabolism in all species, such that the unchanged drug is undetectable in plasma.[41] Most of it is oxidized into albendazole sulfoxide (also known as ricobendazole and albendazole oxide[22][43]) in the liver by cytochrome P450 oxidases (CYPs) and a flavin-containing monooxygenase (FMO),[44] which was discovered later.[45] In humans, the cytochrome P450 oxidases are thought to include CYP3A4[46] and CYP1A1,[41] while those in the rats are thought to be CYP2C6 and CYP2A1.[47]
Oxidation to albendazole sulfoxide by FMO produces R(+) enantiomers, while oxidation the cytochromes and by some enzymes in the gut epithelium produce S(-). Different species produce the R(+) and S(-) enantiomers in different quantities; humans, dogs, and most other species[47] produce the R(+) enantiomer more (with the human AUC ratio being 80:20).[30][34][41] Compared to the S(-) enantiomer, the R(+) has greater pharmacological activity, lasts longer in the bloodstream, is found in higher concentrations in the infected host tissues, and is found in higher concentrations within the parasites themselves.[47][37] Some albendazole is also converted to hydroxyalbendazole, mainly by CYP2J2.[25][48]
For systemic parasites, albendazole acts as a prodrug, while albendazole sulfoxide reaches systemic circulation and acts as the real antihelminthic.[12] Albendazole sulfoxide is able to cross the blood–brain barrier and enter the cerebrospinal fluid at 43% of plasma concentrations; its ability to enter the central nervous system allows it to treat neurocysticercosis.[34]
Albendazole sulfoxide is converted to the inactive albendazole sulfone by cytochrome P450 oxidases, thought to include CYP3A4[34] or CYP2C.[12] Other inactive metabolites include: 2-aminosulfone, ω-hydroxysulfone, and β-hydroxysulfone.[44][30] The major final metabolites that are excreted by humans are:[12]
- methyl [5-(propylsulfonyl-1H-benzimidazol-2-yl)] carbamate,
- methyl [6-hydroxy 5-(n-propylsulfonyl)-1H-benzimidazole-2-yl)] carbamate,
- methyl [5-(n-propylsulfinyl)-1H-benzimidazole-2-yl)] carbamate,
- 5-(n-propylsulfonyl)-1H-benzimidazole-2-yl amine, and
- 5-(n-propysulfinyl)-1H-benzimidazole-2-yl amine.
There are also some minor hydroxylated sulfated or glucuronidated derivatives.[12] No unchanged albendazole is excreted, as it is metabolized too quickly.[2]
In humans, the metabolites are excreted mostly in bile, with only a small amount being excreted in urine (less than 1%) and feces.[2][12] In ruminants, 60–70% of the metabolites are excreted in urine.[26]
Like all benzimidazoles, albendazole has no residual effect, and thus protects poorly against reinfestations.[22]
History
Albendazole, patented in 1975, was invented by Robert J. Gyurik and Vassilios J. Theodorides and assigned to SmithKline Corporation.[49][50] It was introduced in 1977 as an antihelminthic for sheep in Australia, and was registered for human use in 1982.[9][12]
Society and culture

Brand names
Brand names include: Albenza,[28] Alworm, Andazol, Eskazole, Noworm, Zentel, Alben-G, ABZ, Cidazole, Wormnil etc.
Economics
The pharmaceutical company Amedra increased the price after purchasing the rights to the drug, instead of lowering it as generics are predicted to do, drawing criticism from patients' rights advocates.[51]
In 2013, GlaxoSmithKline donated 763 million albendazole tablets for the treatment and prevention of parasitic infections in developing countries, bringing the total to over 4 billion tablets donated since 1998.[52]
Research
Albendazole and related compounds or metabolites like albendazole sulfone (ALB-SO2) exhibit antibacterial effects via an unknown, possibly FtsZ-related, mechanism. It inhibits division of Wolbachia and Mycobacterium tuberculosis, turning them into a long "filament" shape as they grow and fail to divide. Since Brugia malayi relies on symbiotic Wolbachia, this would mean that albendazole is targeting both the worm and its essential symbioant.[53]
Veterinary use
Albendazole is mainly used in cattle and sheep, but has found some use in cats and dogs as well;[23] it is also used in ratite birds for flagellate parasites and tapeworms. It is also used off-label to treat endoparasites in goats and pigs.[1]
| Cattle | Sheep | Others | |
|---|---|---|---|
| Platyhelminthes (flatworms) | |||
| Trematodes | |||
| Dicrocoelium (liver flukes) | D. dendriticum (lancet liver fluke)[57] | D. dendriticum[58] | |
| Fasciola (liver flukes) | F. hepatica | F. hepatica | For F. hepatica and F. gigantica in people[3] |
| Fascioloides (liver flukes) | F. magna[57] | F. magna | Also for F. magna in South American camelids (ex. llama and alpaca)[58] |
| Paragonimus (lung flukes) | — | — | For P. kellicotti in cats and dogs[58] |
| Platynosomum | — | — | For Platynosomum infections in cats |
| Opisthorchiidae | — | — | For Opisthorchiidae infections in cats |
| Cestodes (tapeworms) | |||
| Echinococcus | – | — | For Echinococcus cysts in horses and humans[58][3] |
| Moniezia | M. expansa M. benedini |
M. expansa |
|
| Taenia | T. saginata larvae | — | For T. saginata, T. solium, and T. crassiceps in humans[7][8] and Taenia infections in dogs[59] |
| Thysanosoma | — | T. actinoides | |
| Nematodes (roundworms) | |||
| Ancylostoma | — | – | For Ancylostoma infections in dogs, cats, and humans[58][3] |
| Bunostomum | B. phlebotomum | – | |
| Capillaria | — | — | For causative agents of various forms of capillariasis in cats and dogs (including C. philippinensis, C. hepatica, C. aerophila, and C. plica) and intestinal capillariasis (C. philippinensis) in humans. |
| Chabertia | — | C. ovina | |
| Cooperia | C. oncophora C. punctata |
C. oncophora | |
| Dictyocaulus (lungworm) | D. viviparus | D. filaria | For D. amfieldi infections in horses |
| Filaroides (lungworm) | — | — | For F. hirthi and F. osleri in dogs |
| Haemonchus | H. contortus H. placei |
H. contortus |
|
| Marshallagia | — | M. marshalli | |
| Metastrongylus | — | — | For M. apri in swine |
| Nematodirus | N. spathiger N. helvetianus |
N. spathiger N. filicollis |
|
| Parascaris | — | — | For P. equorum in horses[55] |
| Ostertagia | O. ostertagi | O. circumcincta | For O. bifurcum in humans |
| Oesophagostomum | O. radiatum | O. columbianum | |
| Strongyloides | — | — | For S. stercoralis in dogs and humans[3][58] |
| Strongylus | — | — | For S. equinus in horses[58] |
| Toxocara | — | — | For T. canis infections in dogs[58] and toxocariasis in humans (caused by T. canis and T. cati) |
| Trichostrongylus | T. axei T. colubriformis |
T. axei T. colubriformis |
For any Trichostrongylus infection in humans |
| Trichuris (whipworm) | Most species, but those usually found in cattle are:[60] T. discolor T. globulosa T. ovis |
Most species, but those usually found in sheep are:[60] T. discolor T. globulosa T. ovis |
Albendazole is also used for Trichuris infections in humans (usually T. trichiura, causative agent of trichuriasis), dogs (usually T. vulpis and T. campanula), cats (usually T. serrata and T. campanula), pigs (usually T. suis), and other ruminants (same species as those found in cattle and sheep).[60] |
| Other | |||
| Encephalitozoon | — | — | For E. cuniculi infections (microsporidiosis) in humans and rabbits |
| Giardia | G. lamblia (causative agent of giardiasis) | — | Also treats giardiasis in humans, dogs, and small mammals |
| Leishmania | — | — | Treats leishmaniasis, caused by various species of Leishmania, in dogs |
Albendazole has been used as an antihelminthic and for control of flukes in a variety of animal species, including cattle, sheep, goats, swine, camels, dogs, cats, elephants, poultry, and others.[26][61] Side effects include anorexia in dogs and lethargy, depression, and anorexia in cats,[1] with more than 10% of dogs and cats having anorexia.[27] Of dogs and cats, 1–10% experience elevated liver enzymes, nausea, vomiting, and diarrhea. Less than 1% experience neutropenia or aplastic anemia, though these require a use of at least 5 days.[27] While it is also associated with bone marrow suppression and toxicity in cats and dogs at high doses, albendazole has a higher margin of safety in other species.[23][54] Thus, it is usually only prescribed in cats and dogs when an infection is present that is resistant to the commonly prescribed metronidazole and fenbendazole.[62]
It is extensively used for ruminant livestock in Latin America.[22] It is marketed for this purpose by Zoetis (formerly Pfizer Animal Health) in numerous countries (including the United States and Canada) as Valbazen in oral suspension and paste formulations;[1][23] by Interchemie in the Netherlands and elsewhere as Albenol-100; by Channelle Animal Health Ltd. in the United Kingdom as Albex; and by Ravensdown in New Zealand (as Albendazole). Although most formulations are administered orally, Ricomax (ricobendazole, or albendazole sulfoxide) is administered by subcutaneous injection.
Albendazole has greater bioavailability in ruminants: some albendazole sulfoxide, when released back into the rumen, is reduced to albendazole by the resident microbiota, with a preference of the (+) enantiomer being the substrate.[47][37] Cats and dogs, having no rumen reservoir, sometimes need higher or more frequent doses as compared to ruminants. In dogs, albendazole sulfoxide is detectable in the plasma for less than 12 hours, but in sheep and goats, it remains at measurable levels for around three days.[26]
Meat
The limitations in early pregnancy are due to a limited period during which teratogenic effects may occur. Summarized research data relating to the durations of these preslaughter and early pregnancy periods when albendazole should not be administered are found in US FDA NADA 110-048 (cattle) and 140-934 (sheep). Some data and inferences regarding goats are found in US FDA Supplemental NADA 110-048 (approved January 24, 2008).
Maximum residue limits (MRLs) for albendazole in food, adopted by the FAO/WHO Codex Alimentarius in 1993, are 5000, 5000, 100, and 100 micrograms per kilogram of body weight (μg/kg) for kidney, liver, fat, and muscle, respectively, and 100 μg/L for milk. For analysis purposes, MRLs of various nations may pertain to concentration of a marker substance which has been correlated with concentrations of the administered substance and its metabolized products. For example, in Canada, the marker substance specified by Health Canada is albendazole-2-aminosulfone, for which the MRL in liver of cattle is 200 μg/kg.
There is a 27-day cattle withdrawal time for meat.[23]
References
- 1 2 3 4 5 6 7 Plumb DC (2011). "Albendazole". Plumb's Veterinary Drug Handbook (7th ed.). Stockholm, Wisconsin; Ames, Iowa: Wiley. pp. 19–21. ISBN 978-0-470-95964-0.
- 1 2 3 4 5 6 7 8 "Albenza, (albendazole) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on March 1, 2014. Retrieved February 25, 2014.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 "Albendazole". Drugs.com. The American Society of Health-System Pharmacists. Archived from the original on September 23, 2015. Retrieved August 18, 2015.
- ↑ Australian Government (March 3, 2014). "Prescribing medicines in pregnancy database". Archived from the original on April 8, 2014. Retrieved April 22, 2014.
- ↑ Neonatal Formulary: Drug Use in Pregnancy and the First Year of Life. John Wiley & Sons. 2014. p. 64. ISBN 9781118819593. Archived from the original on 2017-09-08.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- 1 2 3 4 5 Tripathi KD (September 30, 2013). Essentials of Medical Pharmacology. JP Medical Ltd. p. 850. ISBN 978-93-5025-937-5. Archived from the original on September 8, 2017.
- 1 2 3 4 5 Wu JJ (October 18, 2012). Comprehensive Dermatologic Drug Therapy E-Book. Elsevier Health Sciences. p. 137. ISBN 978-1-4557-3801-4. Archived from the original on September 8, 2017.
- 1 2 3 4 5 6 7 8 9 Yaffe SJ, Aranda JV (2010). Neonatal and Pediatric Pharmacology: Therapeutic Principles in Practice. Lippincott Williams & Wilkins. pp. 470–472. ISBN 978-0-7817-9538-8. Archived from the original on 2017-09-08.
- 1 2 "Helminths: Cestode (tapeworm) infection: Albendazole". WHO Model Prescribing Information: Drugs Used in Parasitic Diseases - Second Edition. WHO. 1995. Archived from the original on August 31, 2015. Retrieved August 29, 2015.
- ↑ Horton J (April 2003). "Albendazole for the treatment of echinococcosis". Fundamental & Clinical Pharmacology. 17 (2): 205–212. doi:10.1046/j.1472-8206.2003.00171.x. PMID 12667231. S2CID 221750495.
- 1 2 3 4 5 6 7 8 9 10 11 12 Turner A, Horton J (December 30, 1987). "Albendazole". Logan Turner's Diseases of the Nose, Throat and Ear (10th ed.). CRC Press. pp. 2227–2239. ISBN 978-0-340-92767-0.
- 1 2 3 4 5 6 7 8 "Helminths: Intestinal nematode infection: Albendazole". WHO Model Prescribing Information: Drugs Used in Parasitic Diseases - Second Edition. WHO. 1995. Archived from the original on August 31, 2015. Retrieved August 29, 2015.
- 1 2 3 4 5 Sweet RL, Gibbs RS (2009). Infectious Diseases of the Female Genital Tract. Lippincott Williams & Wilkins. pp. 379, 382–383. ISBN 978-0-7817-7815-2. Archived from the original on 2017-09-08.
- ↑ "Quick thinking saves a life". Alberta College of Pharmacy. 2021. Retrieved June 19, 2021.
- 1 2 3 Gouma DJ (2004). Update Gastroenterology 2004: New Developments in the Management of Benign Gastrointestinal Disorders. John Libbey Eurotext. pp. 144–145. ISBN 978-2-7420-0538-3. Archived from the original on 2017-09-08.
- 1 2 3 4 Finch RG, Greenwood D, Whitley RJ, Norrby SR (November 30, 2010). Antibiotic and Chemotherapy E-Book. Elsevier Health Sciences. p. 101. ISBN 978-0-7020-4765-7.
- 1 2 "Drugs: Albendazole". WHO Model Prescribing Information: Drugs Used in HIV-Related Infections. WHO. 1999. Archived from the original on August 29, 2015. Retrieved August 29, 2015.
- ↑ Francesconi F, Lupi O (January 2012). "Myiasis". Clinical Microbiology Reviews. 25 (1): 79–105. doi:10.1128/CMR.00010-11. PMC 3255963. PMID 22232372.
- ↑ "Albenza New FDA Drug Approval". CenterWatch. Archived from the original on July 11, 2017. Retrieved August 8, 2017.
- 1 2 "Albendazole (Albenza) Use During Pregnancy". Drugs.com. Archived from the original on August 8, 2017. Retrieved August 4, 2017.
- 1 2 3 4 5 Junquera P (July 26, 2015). "Ricobendazole = Albendazole Sulfoxide for Veterinary Use on Cattle, Sheep, Goats, Pig Poultry, Dogs and Cats against roundworms, tapeworms and liver flukes". Parasitipedia. Archived from the original on March 4, 2016. Retrieved October 21, 2015.
- 1 2 3 4 5 6 Papich MG (2007). "Albendazole". Saunders Handbook of Veterinary Drugs (2nd ed.). St. Louis, Mo: Saunders/Elsevier. pp. 8–9. ISBN 978-1-4160-2888-8.
- ↑ Briggs GG, Freeman RK, Yaffe SJ (2011). Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins. p. 31. ISBN 978-1-60831-708-0.
- 1 2 Wu Z, Lee D, Joo J, Shin JH, Kang W, Oh S, et al. (November 2013). "CYP2J2 and CYP2C19 are the major enzymes responsible for metabolism of albendazole and fenbendazole in human liver microsomes and recombinant P450 assay systems". Antimicrobial Agents and Chemotherapy. 57 (11): 5448–5456. doi:10.1128/AAC.00843-13. PMC 3811268. PMID 23959307. Archived from the original on 2015-09-04.
- 1 2 3 4 5 6 Junquera P. "Albendazole toxicity, poisoning, intoxication, overdose, antidote: safety summary for veterinary use on dogs, cats, cattle, sheep, goats, swine and poultry". Parasitipedia. Archived from the original on August 8, 2017. Retrieved July 24, 2017.
- 1 2 3 Wiebe VJ (May 11, 2015). Drug Therapy for Infectious Diseases of the Dog and Cat. John Wiley & Sons. p. 247. ISBN 978-1-118-55747-1.
- 1 2 3 4 5 6 7 8 9 10 "ALBENZA- albendazole tablet, film coated (NDC Code(s): 52054-550-22, 52054-550-28)". DailyMed. February 2013. Archived from the original on September 12, 2015. Retrieved September 7, 2015.
- ↑ Farrar J, Hotez PJ, Junghanss T, Kang G, Lalloo D, White NJ (October 26, 2013). Manson's Tropical Diseases E-Book. Elsevier Health Sciences. p. 807. ISBN 978-0-7020-5306-1.
- 1 2 3 4 5 Jung H, Gonzáles-Esquivel DF (2002). "Pharmacology of Anticysticeral Therapy". In Singh G, Prabhakar S (eds.). Taenia Solium Cysticercosis: From Basic to Clinical Science. CABI. pp. 368–371. ISBN 978-0-85199-839-8.
- ↑ "Albenza (Albendazole) – Warnings and Precautions". Archived from the original on March 2, 2011. Retrieved March 9, 2011.
- ↑ Lanchote VL, Garcia FS, Dreossi SA, Takayanagui OM (June 2002). "Pharmacokinetic interaction between albendazole sulfoxide enantiomers and antiepileptic drugs in patients with neurocysticercosis". Therapeutic Drug Monitoring. 24 (3): 338–345. doi:10.1097/00007691-200206000-00003. PMID 12021623. S2CID 25194606. Archived (PDF) from the original on 2017-08-08.
- ↑ Schipper HG, Koopmans RP, Nagy J, Butter JJ, Kager PA, Van Boxtel CJ (December 2000). "Effect of dose increase or cimetidine co-administration on albendazole bioavailability". The American Journal of Tropical Medicine and Hygiene. 63 (5–6): 270–273. doi:10.4269/ajtmh.2000.63.270. PMID 11421376. S2CID 28550143.
- 1 2 3 4 5 6 7 8 Bennett JE, Dolin R, Blaser MJ (August 28, 2014). Principles and Practice of Infectious Diseases. Elsevier Health Sciences. p. 520. ISBN 978-1-4557-4801-3. Archived from the original on December 7, 2016.
- ↑ Wen H, Zhang HW, Muhmut M, Zou PF, New RR, Craig PS (February 1994). "Initial observation on albendazole in combination with cimetidine for the treatment of human cystic echinococcosis". Annals of Tropical Medicine and Parasitology. 88 (1): 49–52. doi:10.1080/00034983.1994.11812834. PMID 8192515.
- ↑ St Georgiev V (1997). Infectious Diseases in Immunocompromised Hosts. CRC Press. p. 695. ISBN 978-0-8493-8553-7.
- 1 2 3 4 Riviere JE, Papich MG (March 17, 2009). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. pp. 1054, 1062. ISBN 978-0-8138-2061-3. Archived from the original on June 3, 2016.
- ↑ Waller DG, Sampson T (June 4, 2017). Medical Pharmacology and Therapeutics E-Book. Elsevier Health Sciences. p. 616. ISBN 978-0-7020-7190-4.
- ↑ Landmann F (March 2019). Cossart P, Roy CR, Sansonetti P (eds.). "The Wolbachia Endosymbionts". Microbiology Spectrum. American Society for Microbiology. 7 (2): 1–15. doi:10.1128/microbiolspec.bai-0018-2019. PMID 30953430. S2CID 96448885.
- ↑ Hoerauf A, Mand S, Fischer K, Kruppa T, Marfo-Debrekyei Y, Debrah AY, et al. (November 2003). "Doxycycline as a novel strategy against bancroftian filariasis-depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production". Medical Microbiology and Immunology. 192 (4): 211–216. doi:10.1007/s00430-002-0174-6. PMID 12684759. S2CID 23349595.
- 1 2 3 4 5 Dayan AD (May 2003). "Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics". Acta Tropica. Preparing to control Schistosomiasis and Soil-transmitted Helminthiasis in the Twenty-First Century. 86 (2–3): 141–159. doi:10.1016/S0001-706X(03)00031-7. PMID 12745134.
- ↑ Boullata JI, Armenti VT (March 17, 2010). Handbook of Drug-Nutrient Interactions. Springer Science & Business Media. p. 306. ISBN 978-1-60327-362-6.
- ↑ "Ricobendazole | C12H15N3O3S (CID=83969)". PubChem. National Center for Biotechnology Information. October 17, 2015. Archived from the original on March 6, 2016. Retrieved October 21, 2015.
- 1 2 Rawden HC, Kokwaro GO, Ward SA, Edwards G (April 2000). "Relative contribution of cytochromes P-450 and flavin-containing monoxygenases to the metabolism of albendazole by human liver microsomes". British Journal of Clinical Pharmacology. 49 (4): 313–322. doi:10.1046/j.1365-2125.2000.00170.x. PMC 2014938. PMID 10759686.
- ↑ Fargetton X, Galtier P, Delatour P (July 1986). "Sulfoxidation of albendazole by a cytochrome P450-independent monooxygenase from rat liver microsomes". Veterinary Research Communications. 10 (4): 317–324. doi:10.1007/BF02213995. PMID 3739217. S2CID 24053943.
- ↑ Stipanuk MH, Caudill MA (August 13, 2013). Biochemical, Physiological, and Molecular Aspects of Human Nutrition - E-Book. Elsevier Health Sciences. p. 564. ISBN 978-0-323-26695-6.
- 1 2 3 4 Capece BP, Virkel GL, Lanusse CE (September 2009). "Enantiomeric behaviour of albendazole and fenbendazole sulfoxides in domestic animals: pharmacological implications". Veterinary Journal. 181 (3): 241–250. doi:10.1016/j.tvjl.2008.11.010. hdl:11336/95707. PMID 19124257.
- ↑ Karkhanis A, Hong Y, Chan EC (July 2017). "Inhibition and inactivation of human CYP2J2: Implications in cardiac pathophysiology and opportunities in cancer therapy". Biochemical Pharmacology. 135: 12–21. doi:10.1016/j.bcp.2017.02.017. PMID 28237650. S2CID 43456597. Archived from the original on 2019-04-27. Retrieved 2019-09-07.
- ↑ US patent 003915986, Gyurkik, Robert; Theodorides, Vassilios, "Methyl 5-propylthio-2-benzimidazolecarbamate", published October 28, 1975, assigned to SmithKline Corporation
- ↑ US patent 956499, Gyurik R, Theodorides V, "Methods and compositions for producing polyphasic parasiticide activity using methyl 5-propylthio-2-benzimidazolecarbamate", published May 11, 1976, assigned to SmithKline Corporation
- ↑ Greene JA (2015-09-23). "Generic drug price gouging: How Shkreli and other monopolists cornered the market on essential medications". Slate. Archived from the original on 2015-11-06.
- ↑ Gustavsen KM, Bradley MH, Wright AL (October 2009). "GlaxoSmithKline and Merck: private-sector collaboration for the elimination of lymphatic filariasis". Annals of Tropical Medicine and Parasitology. 103 (Suppl 1): S11–S15. doi:10.1179/000349809X12502035776478. PMID 19843393. S2CID 206837136.
- ↑ Serbus LR, Landmann F, Bray WM, White PM, Ruybal J, Lokey RS, et al. (September 2012). "A cell-based screen reveals that the albendazole metabolite, albendazole sulfone, targets Wolbachia". PLOS Pathogens. 8 (9): e1002922. doi:10.1371/journal.ppat.1002922. PMC 3447747. PMID 23028321.
- 1 2 Bowman DD (March 12, 2014). Georgis' Parasitology for Veterinarians - E-Book. Elsevier Health Sciences. p. 282. ISBN 978-1-4557-3988-2.
- 1 2 Junquera P (February 11, 2017). "Albendazole for veterinary use on cattle, sheep, goats, pig poultry, dogs and cats against roundworms, tapeworms and liver flukes". Parasitipedia. Archived from the original on August 8, 2017. Retrieved August 3, 2017.
- ↑ "VALBAZEN- albendazole suspension". DailyMed. US National Library of Medicine. Archived from the original on August 8, 2017. Retrieved August 2, 2017.
- 1 2 Divers TJ, Peek SF (2008). Rebhun's Diseases of Dairy Cattle. Elsevier Health Sciences. p. 238. ISBN 978-1-4160-3137-6. Archived from the original on 2017-09-08.
- 1 2 3 4 5 6 7 8 Junquera P (December 8, 2016). "Albendazole dose for dogs, cats, horses, cattle, sheep, goats, swine and other domestic animals". Parasitipedia. Archived from the original on August 8, 2017. Retrieved August 3, 2017.
- ↑ Webster C (March 2001). Clinical Pharmacology. Teton NewMedia. pp. 91, 142. ISBN 978-1-893441-37-8. Archived from the original on 2017-09-08.
- 1 2 3 Junquera P (December 12, 2016). "Trichuris spp., parasitic whipworms of dogs, cats and livestock - cattle, sheep, goats and pigs: Biology, prevention and control". Parasitipedia. Archived from the original on August 8, 2017. Retrieved August 3, 2017.
- ↑ Fowler ME (October 2, 2006). Biology, Medicine, and Surgery of Elephants. John Wiley & Sons. p. 174. ISBN 978-0-8138-0676-1. Archived from the original on September 8, 2017.
- ↑ Webster C (March 2001). Clinical Pharmacology. Teton NewMedia. p. 142. ISBN 978-1-893441-37-8. Archived from the original on 2017-09-08.
External links
- "Albendazole". Drug Information Portal. U.S. National Library of Medicine.