 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Afobazole |
| Other names | Obenoxazine |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 43.64%, pronounced first-pass effect |
| Metabolism | extensive hepatic |
| Onset of action | 0.85±0.13 hours |
| Elimination half-life | 0.82±0.54 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
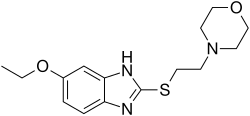
| Formula | C15H21N3O2S |
| Molar mass | 307.41 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |

Fabomotizole (INN;[1] brand name Afobazole) is an anxiolytic drug launched in Russia in the early 2000s. It produces anxiolytic and neuroprotective effects without any sedative or muscle relaxant actions. Its mechanism of action remains poorly defined however, with GABAergic, NGF- and BDNF-release-promoting, MT1 receptor agonism, MT3 receptor antagonism, and sigma agonism suggested as potential mechanisms. Fabomotizole was shown to inhibit MAO-A reversibly and there might be also some involvement with serotonin receptors.[2][3][4][5][6] Clinical trials have shown fabomotizole to be well tolerated and reasonably effective for the treatment of anxiety.[7]
Experiments of mice have shown antimutagenic and antiteratogenic properties.[8]
Fabomotizole has found little clinical use outside Russia and has not been evaluated by the FDA.
See also
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). WHO Drug Information. 26 (1): 63. 2012. Retrieved 21 March 2015.
- ↑ Neznamov GG, Siuniakov SA, Chumakov DV, Bochkarev VK, Seredenin SB (2001). "[Clinical study of the selective anxiolytic agent afobazol]". Eksperimental'naia i Klinicheskaia Farmakologiia. 64 (2): 15–19. PMID 11548440.
- ↑ Silkina IV, Gan'shina TC, Seredin SB, Mirzoian RS (2005). "[Gabaergic mechanism of cerebrovascular and neuroprotective effects of afobazole and picamilon]". Eksperimental'naia i Klinicheskaia Farmakologiia. 68 (1): 20–24. PMID 15786959.
- ↑ Seredin SB, Melkumian DS, Val'dman EA, Iarkova MA, Seredina TC, Voronin MV, Lapitskaia AS (2006). "[Effects of afobazole on the BDNF content in brain structures of inbred mice with different phenotypes of emotional stress reaction]". Eksperimental'naia i Klinicheskaia Farmakologiia. 69 (3): 3–6. PMID 16878488.
- ↑ Antipova TA, Sapozhnikova DS, Bakhtina LI, Seredenin SB (2009). "[Selective anxiolytic afobazole increases the content of BDNF and NGF in cultured hippocampal HT-22 line neurons]". Eksperimental'naia i Klinicheskaia Farmakologiia. 72 (1): 12–14. PMID 19334503.
- ↑ Seredenin SB, Antipova TA, Voronin MV, Kurchashova SY, Kuimov AN (July 2009). "Interaction of afobazole with sigma1-receptors". Bulletin of Experimental Biology and Medicine. 148 (1): 42–44. doi:10.1007/s10517-009-0624-x. PMID 19902093. S2CID 37411324.
- ↑ Medvedev VE, Trosnova AP, Dobrovol'skiĭ AV (2007). "[Psychopharmacotherapy of anxiety disorders in patients with cardio-vascular diseases: the use of aphobazole]". Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova. 107 (7): 25–29. PMID 18379478.
- ↑ Durnev AD, Zhanataev AK, Shreder OV, Seredenin SB (Jan–Feb 2009). "[Antimutagenic and antiteratogenic properties of afobazole]". Eksperimental'naia i Klinicheskaia Farmakologiia. 72 (1): 46–51. PMID 19334511.