 | |
| Pharmacokinetic data | |
|---|---|
| Elimination half-life | 30 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
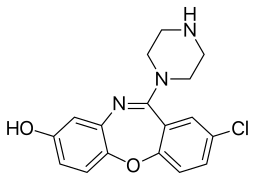
| Formula | C17H16ClN3O2 |
| Molar mass | 329.78 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
8-Hydroxyamoxapine is an active metabolite of the antidepressant drug amoxapine (Asendin). It contributes to amoxapine's pharmacology.[1][2] It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with similar norepinephrine, but more serotonin, reuptake inhibition as its parent compound. It plays a part in balancing amoxapine's ratio of serotonin to norepinephrine transporter blockage.[3]
See also
References
- ↑ Jue SG, Dawson GW, Brogden RN (July 1982). "Amoxapine: a review of its pharmacology and efficacy in depressed states". Drugs. 24 (1): 1–23. doi:10.2165/00003495-198224010-00001. PMID 7049659. S2CID 7279867.
- ↑ Calvo B, García MJ, Pedraz JL, Mariño EL, Domínguez-Gil A (April 1985). "Pharmacokinetics of amoxapine and its active metabolites". International Journal of Clinical Pharmacology, Therapy, and Toxicology. 23 (4): 180–185. PMID 3997304.
- ↑ Midha KK, Hubbard JW, McKay G, Rawson MJ, Hsia D (September 1999). "The role of metabolites in a bioequivalence study II: amoxapine, 7-hydroxyamoxapine, and 8-hydroxyamoxapine". International Journal of Clinical Pharmacology and Therapeutics. 37 (9): 428–38. PMID 10507241.
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
| H1 |
| ||||
|---|---|---|---|---|---|
| H2 |
| ||||
| H3 |
| ||||
| H4 |
| ||||
| |||||
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Classes | |
|---|---|
| Antidepressants (Tricyclic antidepressants (TCAs)) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Anticholinergics | |
| Others |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.